N. Pavone1,*, F. Cammertoni1,*, P. Bruno1, G. Cutrone1,§, G.A. Chiariello1, M. Calabrese1, M. Grandinetti1, M. Nesta1, E. Marzetti1,2, R. Calvani2, R. Gambardella1, A.D. Conserva1, E. Romagnoli1, F. Burzotta1,2, M. Massetti1,2
1. Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Largo Agostino Gemelli 8, 00168, Rome, Italy; 2. Università Cattolica del Sacro Cuore, Largo Francesco Vito 1, 00168 Rome, Italy; * These two authors contributed equally to this work; § this research has been conducted as a part of her Medical Degree final thesis.
Corresponding Author: Natalia Pavone, MD PhD, Department of Cardiovascular Sciences, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Largo A. Gemelli, 8 00168 Rome (Italy), email: natalia.pavone@policlinicogemelli.it, Tel: +390630154814 Fax: +39063015588; E. Marzetti, Università Cattolica del Sacro Cuore, Largo Francesco Vito 1, 00168 Rome, Italy, emanuele.marzetti@policlinicogemelli.it
J Frailty Aging 2024;13(4)501-506
Published online June 21, 2024, http://dx.doi.org/10.14283/jfa.2024.54
Abstract
BACKGROUND: Malnutrition has been variously associated with poor postoperative outcomes. Of note, 10–25 % of cardiac surgery patients are reported to be malnourished.
OBJECTIVES: To assess the impact of nutritional status (evaluated with the Geriatric Nutritional Risk Index – GNRI) on outcomes of older patients undergoing heart valve surgery.
DESIGN: Retrospective, single-center.
SETTING: Cardiac Surgery Unit, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy.
PARTICIPANTS: 448 patients older than 75 years who had undergone isolated, elective heart valve surgery. Patients were divided into low (GNRI≥92; 346 patients) and moderate-to-high (GNRI<92; 102 patients) risk groups of nutrition-related complications.
MEASUREMENTS: Demographic, clinical, and biological variables were retrieved from the institutional Heart Valve Database. GNRI was calculated as follows: [1.489 × serum albumin (g/dL)] + [41.7 × actual body weight (kg) / ideal body weight (kg)]. Operative and postoperative outcomes were compared between GNRI groups. Survival at 3 years follow-up was analyzed using the Kaplan-Meier method and log-rank test. Cox regression was used to identify variables associated with survival.
RESULTS: Mortality at 30 days did not differ between groups (0.98% vs 0.58% for GNRI < 92 and GNRI ≥ 92, respectively; p=0.54). Those with a GNRI < 92 required more frequently dialysis (2.9% vs 0.3%, p=0.04), inotropes (33.3% vs 22.8%, p=0.04), red blood cells transfusions (63.7% vs 19.9%, p<0.01), and longer mechanical ventilation support (12 ± 2 vs 6 ± 1.5 hours, p=0.03). Intensive care unit (4.7 ± 0.9 vs 1.6 ± 0.8 days, p=0.05) and total postoperative hospital (11.1 ± 1.9 vs 5.2 ± 1.5 days, p=0.05) stays were significantly longer in the GNRI < 92 group.
CONCLUSION: A poor nutritional status may increase morbidity and prolong hospitalization after cardiac surgery. GNRI might improve risk assessment and should be integrated into traditional surgical risk models to offer tailored care to older patients.
Key words: Heart valve surgery, geriatric nutritional risk index, nutritional assessment, frailty.
Introduction
Over the last decade, the number of older patients (i.e. ≥ 75 years) referred to cardiac surgery has increased noticeably (1). In this age group, comorbidities and frailty are common and have both been associated with poor surgical outcomes (2). To date, the Society of Thoracic Surgeons (STS) score and the European System for Cardiac Operative Risk Evaluation (EuroSCORE) are considered the gold standard for assessing risk of morbidity and mortality after cardiac surgery (3). However, both these models do not take into consideration certain health issues (low physical performance, disability, and frailty) that are common in older patients (4-5). Hence, their value and applicability to the contemporary cardiac surgery era are questionable.
Frailty is a geriatric syndrome that reflects a state of decreased homeostatic reserve and increased vulnerability to stressors, due to cumulative declines across multiple physiologic systems (6). Current guidelines recommend frailty assessment in addition to traditional scores for a more accurate risk stratification in older patients referred to cardiac surgery (7).
Regardless of the operational definition used for frailty assessment, nutritional status is an important criterion (8). Malnutrition, either overt or subclinical, has been associated with higher morbidity and mortality in various clinical settings, including cardiac surgery (9-10). Furthermore, malnutrition is highly prevalent in older adults, ranging from 20% to 78% depending on the scale used (11-12).
The Geriatric Nutritional Risk Index (GNRI) (13) is a simple tool that has shown to predict the risk of nutrition-related complications and mortality (10, 14-16).
In this study, we retrospectively explored the impact of nutritional status, evaluated with the GNRI, on outcomes of a contemporary cohort of patients who underwent isolated, elective heart valve surgery.
Methods
Study Population
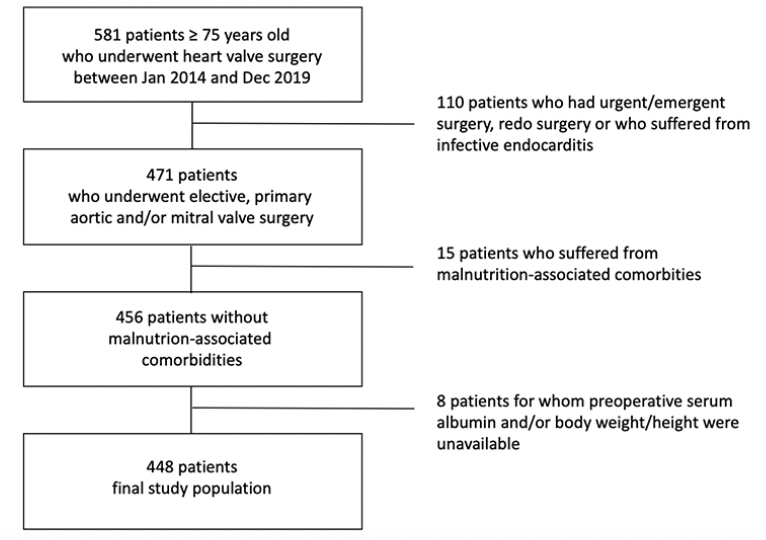
Between January 2014 and December 2019, 581 patients older than 75 years underwent isolated heart valve surgery at our hospital. After exclusion of emergent/urgent cases, re-operative surgery, and active endocarditis, 471 patients who underwent aortic and/or mitral valve surgery were eligible. As we aimed to evaluate the impact of age-related nutritional status on outcomes, we excluded those who suffered from malnutrition-associated comorbidities such as malignancy, severe gastro-intestinal or liver disease. Finally, patients for whom preoperative serum albumin values or body weight and height measurements were inaccurate or unavailable were also excluded, thus obtaining a final study sample of 448 patients (Figure 1).
The local Heart Team decided about surgical indication on the basis of patients’ general health status, calculated surgical risk, and anatomical feasibility. Surgery was performed through a conventional or minimally invasive upper sternotomy approach, as previously described (17). Preoperative, intraoperative, and post-operative data were gathered retrospectively from our institutional Heart Valves Database. Study-dedicated postoperative follow-up was carried out by two cardiac surgery residents through phone interviews. The study received approval from the local Institutional Review Board. Due to the retrospective design, informed consent was waived.
Nutritional and Frailty assessment
In line with literature, GNRI score was calculated as follows: GNRI = [1.489 × serum albumin (g/dL)] + [41.7 × actual body weight (kg) / ideal body weight (kg)]. The ideal body weight was calculated using the Lorentz’s formula (13).
Conventionally, a GNRI score ≥ 92 indicates patients at low risk of nutrition-related complications, whereas scores between 82 and 91 or ≤ 82 identify those at moderate and high risk, respectively (13).
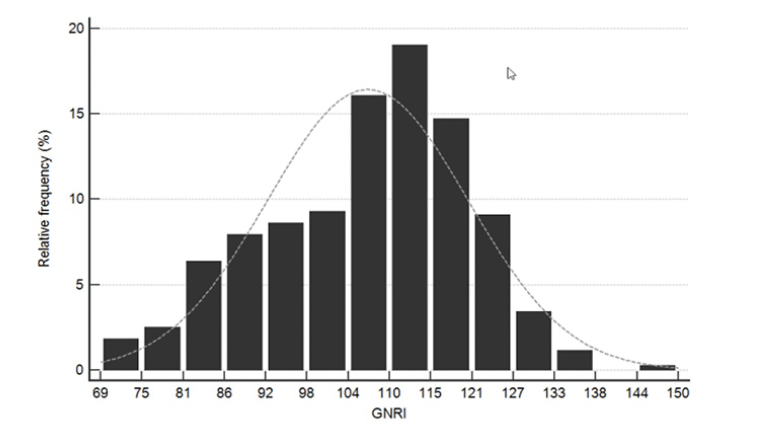
Due to the small number of patients with GNRI ≤ 82 in our cohort (16 patients, 3.6%), patients were divided into a low (GNRI ≥ 92; 346 patients, 77.2%) and a moderate-to-high risk group (GNRI < 92; 102 patients, 22.8%). The distribution of GNRI scores in the study sample is shown in Figure 2.
Collected Data and Outcomes
Starting from 2012, data of patients undergoing heart valve surgery have been registered in a dedicated database. For the purpose of this study, we retrieved the following information: demographic data, cardiological history and comorbidities, results of preoperative routine laboratory tests, echocardiogram report, calculated surgical risk scores, and surgical report. Postoperative outcomes were: 30-day mortality, duration of mechanical ventilation (hours), use of inotropic drugs, postoperative stroke and myocardial infarction, postoperative bleeding requiring surgical revision, postoperative atrial fibrillation, acute kidney injury requiring hemodialysis, permanent pacemaker implantation, red blood cells transfusion, deep sternal wound complications, and intensive care unit and hospital length of stay (days). At follow-up, a pre-specified questionnaire investigating mortality, symptoms relapse, need for early (< 12 months) reoperation and re-hospitalization for heart failure was administered and data were registered by two cardiac surgery residents.
Statistical Analysis
The Kolmogorov–Smirnov test was used to check the normality/skewness of continuous data before analysis. Continuous variables were shown as mean ± standard deviation if normally distributed or median and interquartile range otherwise. Absolute numbers and percentages were used to summarize categorical variables. Continuous variables were compared using independent samples t-test or Mann Whitney U tests, as appropriate. Categorical variables were compared by Pearson chi-square or Fisher’s exact test, as appropriate. All tests were two-sided with a type I error significance level of 0.05. Missing data were replaced by the mean if their percentage was below 5% for the examined variable. If the number of missing data was > 5%, a listwise deletion method was adopted. Survival was analyzed using the Kaplan-Meier method and groups were compared using log-rank test. Variables with a p value < 0.1 and those commonly considered as meaningful in literature were then analyzed using stepwise Cox regression to determine which of them independently predicted decreased survival time. Data analysis was performed with IBM SPSS Statistics version 19.0 (SPSS Inc, Chicago, IL, USA).
Results
Baseline Patients Characteristics and Surgery Data
Baseline characteristics and operative data of the study sample according to GNRI score are shown in Table 1.
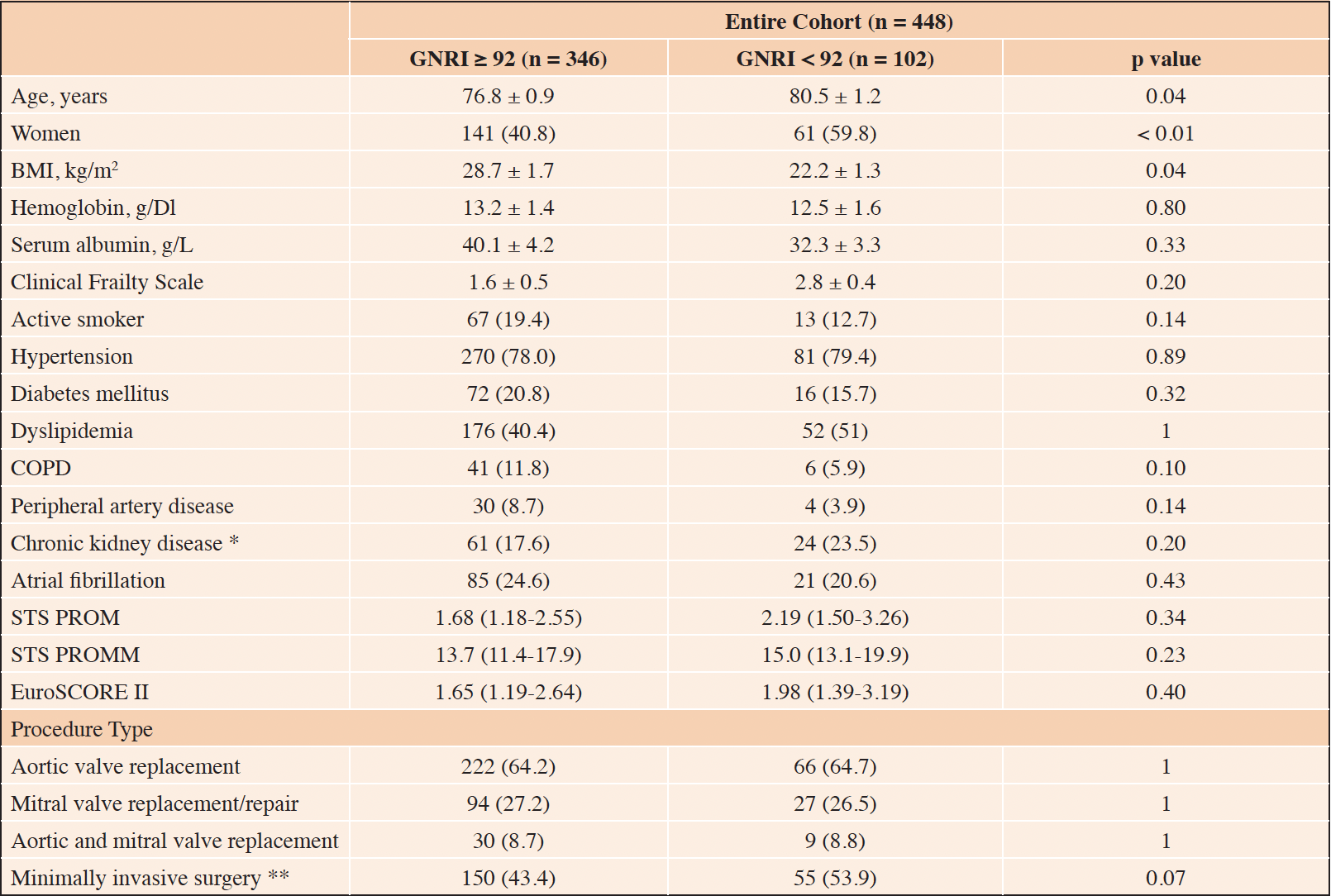
Table 1. Baseline characteristics and operative data stratified by Geriatric Nutritional Risk Index (GNRI) score
Continuous variables were reported by mean ± standard deviation if normally distributed or median (interquartile range) otherwise. Categorical variables were described as absolute value (percentage). BMI = Body Mass Index; COPD = Chronic Obstructive Pulmonary Disease; GNRI = Geriatric Nutritional Risk Index; STS PROM Score = Society Thoracic Surgeon risk of mortality; STS PROMM Score = Society Thoracic Surgeon risk of morbidity and mortality; * Glomerular filtration rate (estimated with the Cockcroft and Gault formula) < 50 ml/min off dialysis or on dialysis; *** All minimally invasive procedures were performed through an upper partial sternotomy
Patients with GNRI < 92 were significantly older (80.5 ± 1.2 vs. 76.8 ± 0.9 years, p = 0.04), were more frequently women (59.8 % vs. 40.8 %, p < 0.01), and had lower BMI (22.2 ± 1.3 vs. 28.7 ± 1.7 kg/m2, p = 0.04) than those with GNRI ≥ 92. Cardiovascular risk factors and comorbidities were comparable between groups. Accordingly, the EuroSCORE II (1.98 [1.39-3.19] vs. 1.65 [1.19-2.64], p = 0.40) and the STS score (2.19 [1.50-3.26] vs. 1.68 [1.18-2.55], p = 0.34) did not differ between groups.
Isolated aortic valve replacement was the most common procedure (288 patients; 64.3%), followed by isolated mitral valve replacement/repair (121 patients; 27%) and combined aortic and mitral surgery (39 patients; 8.7%), with no difference between GNRI groups. Although not reaching statistical significance, a minimally invasive approach was more frequently chosen for patients with GNRI < 92 (53.9% vs. 43.4%, p = 0.07).
Postoperative Outcomes
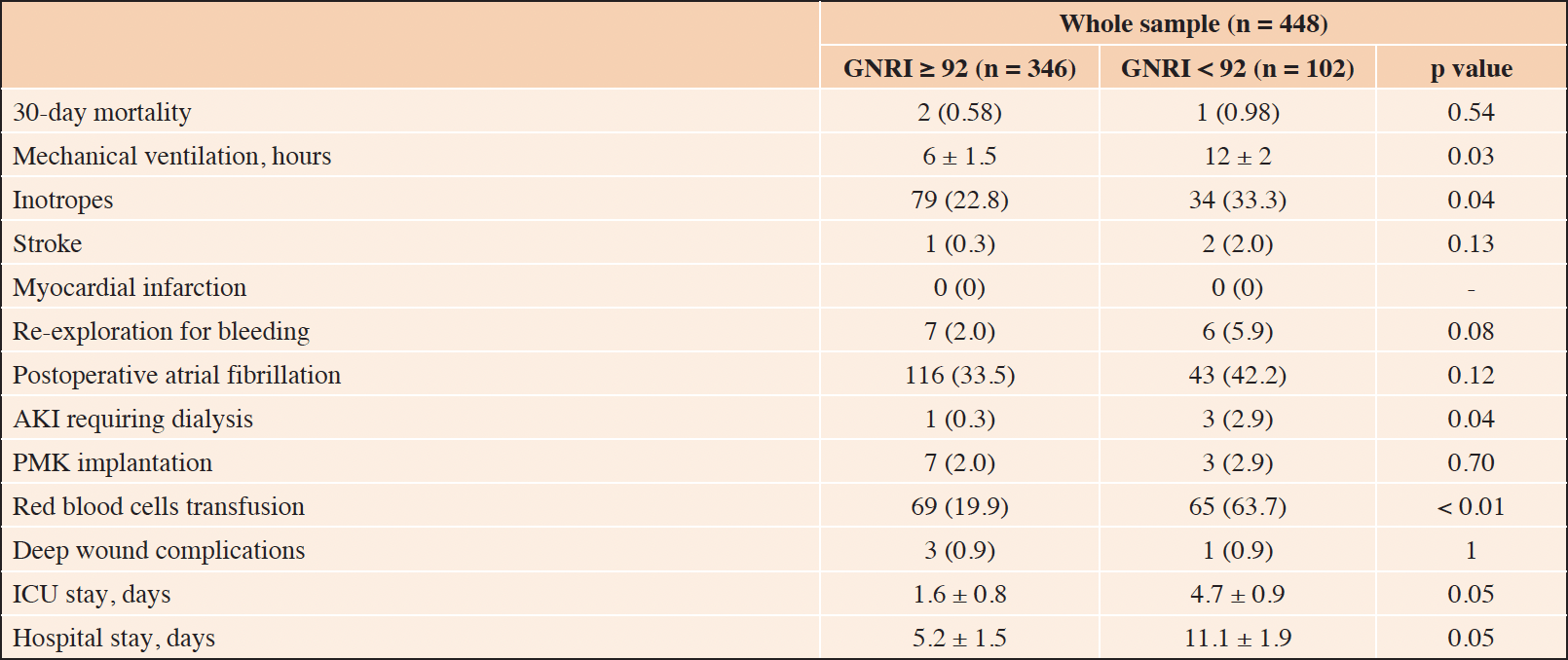
Thirty-day mortality was similar between GNRI groups (0.98% vs. 0.58% for GNRI < 92 and GNRI ≥ 92, respectively; p = 0.54) (Table 2).
Continuous variables were reported by mean ± standard deviation if normally distributed or median (interquartile range) otherwise. Categorical variables were described as absolute value (percentage). AKI = Acute kidney injury; GNRI = Geriatric Nutritional Risk Index; PMK = pacemaker; ICU = Intensive care unit.
Instead, postoperative acute kidney injury requiring dialysis was more common in patients with GNRI < 92 (2.9% vs. 0.3%, p = 0.04). Similarly, patients in the GNRI < 92 group more frequently needed red blood cells transfusions (63.7% vs. 19.9%, p < 0.01) and inotropes for a postoperative cardiac low-output state (33.3% vs. 22.8%, p = 0.04). Mechanical ventilation support was longer for patients in the GNRI < 92 group (12 ± 2 vs. 6 ± 1.5 hours, p = 0.03). Intensive care unit (4.7 ± 0.9 days vs. 1.6 ± 0.8 days, p = 0.05) and total hospital stays (11.1 ± 1.9 days vs. 5.2 ± 1.5 days, p = 0.05) were significantly longer in patients in the GNRI < 92 group.
Follow-up
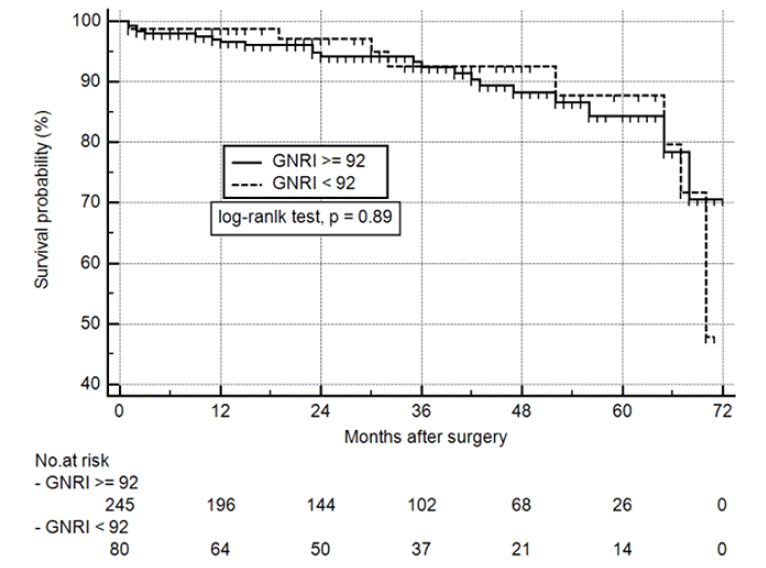
Overall, 325 patients (72.5%) had postoperative follow-up, with a median time of 32.8 months (30.3 – 35.3) vs. 34.0 (29.2 – 38.8) for GNRI ≥ 92 and GNRI < 92, respectively. For the remaining 123 patients, contact information was incorrect or missing (60.2%), patients did not answer (25.2%), or declined to participate (14.6%). As shown in Figure 3, survival was 98.8%, 97%, and 92.6% vs. 96.5%, 94.2%, and 92.4% at 12, 24 and 36 months, respectively for GNRI < 92 vs. GNRI ≥ 92, with no differences between groups (log-rank test p = 0.89).
Stepwise Cox regression analysis demonstrated that preoperative chronic kidney disease (hazard ratio 1.62, 95% CI 1.34 – 1.78, p < 0.01), chronic obstructive pulmonary disease (hazard ratio 1.52, 95% CI 1.18 – 1.98, p = 0.03), and hemoglobin (1 g/dL decrease hazard ratio ratio 1.33, 95% CI 1.08 – 1.69, p = 0.02) were independent predictors of mortality at follow-up (Supplemental Table).
Discussion
The growing number of older patients referred to cardiac surgery is leading to a turning point. This changing population has new and unique features, such as frailty, that are not included in the traditional models used for risk scores calculation. Frailty has been proven to significantly influence surgical and non-surgical outcomes (2). As a consequence, the applicability of traditional risk assessment models to the current surgical population has been questioned (18). Chronic undernutrition is part of the constellation of alterations that define frailty and it is common in older patients, as its prevalence can vary from 20 to 78% depending on the scale used (11-12). Regardless of frailty, previous reports have highlighted the possibility that nutritional status may be useful for risk stratification but 1) an evaluation of patient’s nutrition-related risk of complications is not routinely performed and 2) doubts about the best and simplest tool to assess patient’s nutritional status still exist.
In this retrospective, single-center study we evaluated the impact of baseline nutritional status on outcomes in a sample of older patients undergoing isolated, elective heart valve surgery. Nutritional status can be measured in many different ways. The Mini Nutritional Assessment is a tool that has been recommended for detecting the risk of undernutrition in older adults. It includes a dietary questionnaire and physical and mental aspects that frequently affect the nutritional status of frail older patients (19). However, this tool does not include biological indicators and it may easily be biased in hospitalized older patients (13). Serum albumin alone was found to be inadequate to describe nutritional status because it is influenced by inflammation and hydration (20). The combination of serum albumin and weight loss has been shown to yield a higher prognostic value than serum albumin or body weight alone (13).
The GNRI has been proposed as a prognostic nutritional index able to quantify the risk of nutrition-related morbidity and mortality in older patients (13). It was developed as an adaptation of the Nutritional Risk Index (21) by replacing usual weight, which is difficult to determine in older adults, with ideal weight calculated with the Lorentz formula. In their study, Bouillanne et al. (13) demonstrated that the risk of mortality, infective complications, and bedsores was higher in older patients with low GNRI admitted to a geriatric rehabilitation care unit. Afterwards, other groups tested GNRI in different clinical settings, including cardiovascular disease (15-16). Shibata et al. (15) reported GNRI to predict worse survival after transcatheter aortic valve implantation in a large Japanese multicenter registry including more than 1600 patients. Authors found that 30-day mortality increased significantly across GNRI ≥ 92, GNRI 82 – 92 and GNRI ≤ 82 groups (0.9%, 2.3%, and 6.8%, respectively, p < 0.001). Similarly, 1-year all-cause mortality rates were significantly higher in the lower GNRI groups (16.4% and 36.4%, p < 0.001 for GNRI 82-92 and GNRI ≤82 respectively), although this difference was mainly driven by non-cardiovascular deaths.
More recently, Gürbak et al. (16) found GNRI ≤ 102.5 to be associated with higher 30-day, 1-year and follow-up all-cause mortality in 150 older patients who underwent surgical aortic valve replacement.
Interestingly, our results showed that GNRI < 92 was not associated with increased risk of 30-day mortality nor was it with mid-term mortality after isolated, elective mitral and/or aortic valve surgery. This opposing finding may have two possible reasons. Firstly, Shibata et al. (15) reported results from a large cohort of patients who underwent transcatheter aortic valve implantation. Surgical patients are different from those who are referred to percutaneous procedures. Indeed, 85% of patients included in the Japanese registry were older than 80 years. Moreover, 29%, 47.7%, and 59.1% of patients in the GNRI ≥ 92, GNRI 82 – 92 and GNRI ≤ 82 groups had STS score > 8%. Conversely, even if Gürbak et al. (16) described outcomes of patients who had heart valve surgery, their study population was different as it included younger patients who had isolated aortic valve replacement. These findings suggest that GNRI performance varies according to the type of heart valve disease. However, Cho et al. (22). recently concluded that malnutrition, identified by Controlling Nutritional Status score, Prognostic Nutritional Index and GNRI, was significantly associated with greater 1-year mortality in a retrospective cohort of 1927 patients undergoing different types of heart valve surgery (22).
Secondly, it must be acknowledged that the number of patients with GNRI < 82 in our study was small (3.6%). Although this group of patients is under-represented in many other previous studies, as patients with severe malnutrition are rarely considered for percutaneous or surgical procedures, this may have biased results.
On the other hand, patients with GNRI < 92 had more frequently acute kidney injury requiring dialysis, red blood cells transfusions, inotropes, and prolonged mechanical ventilation. Of note, this difference in postoperative complications was not predicted by STS score or EuroSCORE II, confirming that both these models have limitations when used in older, malnourished patients.
Similar results were found by Unosawa et al. (10) in a retrospective analysis of 287 patients who underwent elective cardiac surgery (aortic, valvular, or coronary surgery). Authors described more frequent pneumonia, prolonged mechanical ventilation (> 72 hours), and postoperative bedridden state in the malnourished group. Although other postoperative complications did not differ (new dialysis, stroke, sepsis, sternal osteomyelitis), longer intensive care unit and hospital stays were reported for patients with GNRI < 91. This prolonged hospitalization may be the consequence of a higher rate of postoperative complications as well as delayed rehabilitation, as highlighted by Ogawa et al. (23). In our study, intensive care unit and hospital stays were on average 3 and 6 days longer, respectivly, in patients with GNRI < 92.
Limitations
Our study has several limitations. Firstly, the small number of patients with very low (< 82) GNRI scores limited the possibility to recognize the outcomes of this high-risk group. Secondly, we included both mitral and aortic valve patients and this mixed population may have biased the outcomes. Also, due to the limited number of events, our study was underpowered to detect clinically meaningful differences in outcomes between patients with preserved vs. low GNRI scores. Finally, we limited our analyses to postoperative mortality and morbidity, and we did not consider other outcomes, such as functional recovery or cost-effect impact, of poor preoperative nutritional status.
Conclusions
Findings from this study support the notion that a poor nutritional status may increase morbidity in older patients and prolong hospitalization after cardiac surgery. Since malnutrition is potentially amenable for corrective interventions, the identification of patients with poor nutritional status could prompt the design of interventions to improve postoperative outcomes.
Therefore, the inclusion of GNRI score in the preoperative evaluation of older patients eligible for heart valve surgery might improve risk assessment, allow personalized interventions, and reduce postoperative morbidity. Adequately powered studies are warranted to establish whether the GNRI also improves mortality risk prediction.
Conflicts of Interest: On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Funding: The work was supported by the Ministero della Salute – Ricerca Corrente 2024. Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Ethical standards: The study was conducted in compliance with the principles laid down in the Declaration of Helsinki. The protocol was approved by the Ethics Committee of the Fondazione Policlinico Universitario A. Gemelli IRCCS / Università Cattolica del Sacro Cuore (Rome, Italy).
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Nishimura RA, O’Gara PT, Bavaria JE, et al. 2019 AATS/ACC/ASE/SCAI/STS Expert Consensus Systems of Care Document: A Proposal to Optimize Care for Patients With Valvular Heart Disease: A Joint Report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2019 28;73:2609-2635.doi:10.1016/j.jacc.2018.10.007
2. Panayi AC, Orkaby AR, Sakthivel D, et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am J Surg. 2019;218:393-400. doi:10.1016/j.amjsurg.2018.11.020
3. Sullivan PG, Wallach JD, Ioannidis JP. Meta-Analysis Comparing Established Risk Prediction Models (EuroSCORE II, STS Score, and ACEF Score) for Perioperative Mortality During Cardiac Surgery. Am J Cardiol. 2016 15;118:1574-1582. doi:10.1016/j.amjcard.2016.08.024
4. Taleb Bendiab T, Brusset A, Estagnasié P, Squara P, Nguyen LS. Performance of EuroSCORE II and Society of Thoracic Surgeons risk scores in elderly patients undergoing aortic valve replacement surgery. Arch Cardiovasc Dis. 2021;114:474-481.doi: 10.1016/j.acvd.2020.12.004.
5. Leontyev S, Walther T, Borger MA, et al. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann Thorac Surg. 2009;87:1440-1445. doi: 10.1016/j.athoracsur.2009.01.057.
6. Cesari M, Calvani R, Marzetti E. Frailty in Older Persons. Clin Geriatr Med. 2017;33:293-303. doi: 10.1016/j.cger.2017.02.002
7. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022 12;43:561-632. doi:10.1093/eurheartj/ehab395.
8. Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millán-Calenti JC. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017;17:108. doi: 10.1186/s12877-017-0496-2.
9. Wells JL, Dumbrell AC. Nutrition and aging: assessment and treatment of compromised nutritional status in frail elderly patients. Clin Interv Aging. 2006;1:67-79. doi: 10.2147/ciia.2006.1.1.67.
10. Unosawa S, Taoka M, Osaka S, et al. Is malnutrition associated with postoperative complications after cardiac surgery? J Card Surg. 2019;34:908-912. doi: 10.1111/jocs.14155
11. Sullivan DH, Bopp MM, Roberson PK. Protein-energy undernutrition and life-threatening complications among the hospitalized elderly. J Gen Intern Med. 2002;17:923-932. doi: 10.1046/j.1525-1497.2002.10930
12. Persson MD, Brismar KE, Katzarski KS, Nordenström J, Cederholm TE. Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc. 2002;50:1996-2002. doi:10.1046/j.1532-5415.2002.50611.x
13. Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777-783. doi: 10.1093/ajcn/82.4.777.
14. Wada H, Dohi T, Miyauchi K, et al. Prognostic Impact of the Geriatric Nutritional Risk Index on Long-Term Outcomes in Patients Who Underwent Percutaneous Coronary Intervention. Am J Cardiol. 2017;119:1740-1745. doi: 10.1016/j.amjcard.2017.02.051
15. Shibata K, Yamamoto M, Kano S, et al. Importance of Geriatric Nutritional Risk Index assessment in patients undergoing transcatheter aortic valve replacement. Am Heart J. 2018;202:68-75. doi: 10.1016/j.ahj.2018.04.021.
16. Gürbak İ, Güner A, Güler A, et al. Prognostic influence of objective nutritional indexes on mortality after surgical aortic valve replacement in elderly patients with severe aortic stenosis (from the nutrition-SAVR trial). J Card Surg. 2021;36:1872-1881.doi: 10.1111/jocs.15434
17. Bruno P, Cammertoni F, Rosenhek R, et al. Improved Patient Recovery With Minimally Invasive Aortic Valve Surgery: A Propensity-Matched Study. Innovations (Phila). 2019;14:419-427. doi: 10.1177/1556984519868715
18. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222-228. doi:10.1161/CIRCOUTCOMES.111.963157
19. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415-421. doi: 10.1016/s0261-5614(03)00098
20. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. 2001;20:271-273. doi: 10.1054/clnu.2001.0439
21. Buzby GP, Williford WO, Peterson OL, et al. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: the rationale and impact of previous clinical trials and pilot study on protocol design. Am J Clin Nutr. 1988;47:357-365. doi: 10.1093/ajcn/47.2.357
22. Cho JS, Shim JK, Kim KS, Lee S, Kwak YL. Impact of preoperative nutritional scores on 1-year postoperative mortality in patients undergoing valvular heart surgery. J Thorac Cardiovasc Surg. 2022;164:1140-1149 doi: 10.1016/j.jtcvs.2020.12.099.
23. Ogawa M, Izawa KP, Satomi-Kobayashi S, et al. Poor preoperative nutritional status is an important predictor of the retardation of rehabilitation after cardiac surgery in elderly cardiac patients. Aging Clin Exp Res. 2017;29:283-290. doi: 10.1007/s40520-016-0552-3.
The Author(s) 2024