F. Forsyth1, C.L. Soh2, N. Elks2, H. Lin2, K. Bailey3, S. Rowbotham4, J. Mant1, P. Hartley1, C. Deaton1
1. Primary Care Unit, Department of Public Health & Primary Care, University of Cambridge, Cambridge, UK; 2. University of Cambridge School of Clinical Medicine, Cambridge, UK; 3. Head of Nursing Cardiac Services, Manchester University NHS Foundation Trust (MFT), Wythenshawe Hospital, Manchester, UK; 4. Department of Physiotherapy, The Queen Elizabeth Hospital King’s Lynn NHS Foundation Trust, King’s Lynn, UK.
Corresponding Author: Faye Forsyth, MSc, Doctoral Student, Primary Care Unit, Department of Public Health & Primary Care, University of Cambridge, Cambridge, CB2 0SR, UK. Fc349@cam.ac.uk; @forsyth_faye; ORCID ID: https://orcid.org/0000-0003-2107-3690
J Frailty Aging 2024;13(4)341-348
Published online March 26, 2024, http://dx.doi.org/10.14283/jfa.2024.28
Abstract
BACKGROUND: Exercise is efficacious in older adults, including those with multi-morbidity. However, the optimum mode is not known and there are conflicting findings as regards the types of exercises to recommend. It is postulated that multi-component exercise interventions better meet the needs of older adults who experience multi-morbidity as they more holistically address the range of functional problems they may experience. To date, no review has explored and described in detail what multi-component exercise interventions have been tested in older adults with multi-morbidity.
OBJECTIVES: To explore the number and types of exercises included within multi-component exercise interventions that have been tested in older adults with multi-morbidity. Secondary objectives were to explore the rationale for selecting particular exercise components within the intervention design and to describe the characteristics of the exercise program.
DESIGN: Systematic review and narrative synthesis.
RESULTS: Database searches yielded 51,001 articles; following screening 138 unique interventions were retained for analysis. Across studies, 22 different multi-component combinations were identified, and there was marked variation in frequency, intensity and duration. Few studies describe characteristics that are in line with the preferences or needs of older adults with multi-morbidity. Exercise design decisions were most frequently judged to be based on practitioner intuition/local practice.
CONCLUSION: There is substantial heterogeneity within multi-component exercise interventions; which has significant implications for meta-analysis of effects. Interventions do not frequently appear to consider the abilities or needs of those with multi-morbidity, nor do they seem to be attuned to the participation barriers they experience.
Key words: Systematic review, older adults, multi-morbidity, exercise interventions
Key words: Type 2 diabetes, frailty, dementia.
Introduction
Exercise interventions effectively improve important outcomes in older adults (1), including in those who have multiple coexisting health conditions (multi-morbidity) (2). However, debate continues around optimal mode of exercise, particularly when both older age and multiple conditions are present (3). People experiencing multi-morbidity report significant burdens in relation to the consequence of their diseases (burden of illness and symptoms)(4) and its management (burden of treatment) (5); which interact to create substantial complexity (6). Such complexity is not easily managed within current healthcare systems, which are typically single-disease focussed (7). As a result, outcomes remain poor (8).
Exercise has the potential to modify both burden of illness and burden of treatment, due the multi-system effects it exerts (9). Further, exercise is generally acceptable to older adults, particularly if the exercise program has been designed with their capabilities and needs in mind (10). Multi-component exercise designs are hypothesised to offer more benefit than single-component interventions for older adults with multi-morbidity as they can better accommodate and address the range of deficits that can accompany aging and accumulation of disease (11, 12). Therefore, despite limited and uncertain evidence (13), the World Health Organisation (WHO) recommends inclusion of varied multi-component physical activities for older adults and those living with chronic conditions and/or disability (14).
When compared against other types of exercise interventions within meta-analyses however, multi-component interventions have not been identified as more efficacious in either older adults or those with multi-morbidity (1, 2, 15, 16). An appraisal of these reviews indicates that multi-component interventions are often grouped together as a sub-category (multimodal (1), multi-component (2), multi-component or combined interventions (15), exercise rehabilitation (17)); with little consideration of within-category heterogeneity. Therefore, important differences in the exercise types included within individual studies may be being ignored, which could lead to misinterpretation.
Rationale and Objectives
On the basis of previous reviews, exercise interventions improve important outcomes in older adults with multi-morbidity (2, 17-19). However, due to divergence in findings as to what generates the largest effects, it is less clear what types of exercises should be included. Common to all previous reviews, was the category ‘multi-component’, ‘multi-modal’ or ‘combined’ exercises interventions, which was statistically compared as an exercise sub-category within meta-analyses (1, 2, 15). There was little focus on the specific types and combinations of exercise within the group ‘multi-component’ and no consideration of the potential impact of this heterogeneity within analyses. This review sought to describe the number and types of exercises included within multi-component exercise interventions that have been tested in older adults with multi-morbidity, as a means of highlighting this heterogeneity.
Methods
The Cochrane Handbook for Systematic Reviews of Interventions (20), and the SWiM (Synthesis without meta-analysis in systematic reviews) items (21), guided the conduct of this review. The subsequent report follows PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (22).
Protocol and Registrations
The review was registered on the International Prospective Register of Systematic Reviews (ID: CRD42020209672).
Eligibility criteria
We considered experimental or quasi-experimental (pre-test post test) primary research, published in the English language. Eligibility according to the PICO (Population, Intervention, Comparator, Outcome) framework are described in detail below.
Population
Studies had to include older adults with multi-morbidity. We characterized older adults as those ≥ 60 years of age; studies where the mean age was ≥60 years were considered eligible. We defined multi-morbidity as people with ≥2 health conditions that require complex and on-going care, in line with the WHO and a recent systematic review of reviews exploring definitions and measures of multi-morbidity (23, 24).
To ensure our scope was broad enough to capture the diversity of studies testing exercise interventions in older adults with multi-morbidity without being explicitly labelled as such, we considered studies that reported exercise interventions in older adults. A substantial body of epidemiological data supports the assertion that multi-morbidity is highly prevalent in older adults across the world (25).
To balance concerns about possible reduced applicability by including participants who may not exactly match the eligibility criteria, we carefully examined inclusion criteria and descriptive statistics for the presence of multi-morbidity within the sample. We applied the common-sense strategy described in the Cochrane Handbook whereby ‘eligibility decisions keep faith with the objectives of the review’ (20). Therefore, populations were considered to be multi-morbid if: mean comorbidities or mean disease counts were greater ≥2; the Charlson Comorbidity Index score was ≥2; baseline characteristics suggested the sample was multi-morbid (e.g. greater than 50% of the sample had 2 or more concurrent conditions). If these criteria could not be satisfied, studies were excluded on the basis that the ‘sample does not clearly meet inclusion criteria’.
Intervention
Any multi-component exercise programmes (i.e. involved ≥2 types of exercises) were included. We used the WHO definition of exercise (14): “a subcategory of physical activity that is planned, structured, repetitive, and purposeful in the sense that the improvement or maintenance of one or more components of physical fitness is the objective”. Providing the aims of the study intervention were the “improvement or maintenance of one or more components of physical fitness” and the physical activity was “structured, repetitive, and purposeful”, the intervention was defined as ‘exercise’ as opposed to ‘physical activity’. Studies that did not provide sufficient data to characterise interventions were excluded as ‘programme characteristics not well enough described’. Studies targeting an isolated or highly specific complication were excluded, as were studies offering advice only.
Descriptions of interventions were assessed by two blinded researchers and exercises were coded based on exercises described. Interventions could be of any frequency, intensity and duration; they could also be combined with other non-exercise components and conducted in any setting (care facility, community).
Comparison
No exclusions were made based on randomisation/comparator group. Studies that did not have a control group (e.g. pre-test post-test) had to have repeated outcome measures (baseline and follow-up).
Outcomes
No study outcomes were considered in this analysis. The outcome of interest in the review was number and type of exercise components, and factors related to the exercise prescription which are described in section 5.3.
Information Sources & Search
Searches, performed with the support of an information specialist, were conducted in seven bibliographic databases (MEDLINE [via Ovid], Embase [Via OVID], EMCARE [via Ovid], AMED [via Ovid], CINAHL [via EBSCO], Cochrane Library, Web of Science). Database selection was based on recommendations described by Bramer et al (26); and searches spanned the period from database inception to 16 November 2020. Reference list and citation searches of included articles and previous systematic reviews were conducted. Details of search strategies can be found in Supplementary Table 1.
Study Selection
The web-based platform Rayyan (27) was used to screen titles and abstracts. Two reviewers screened results returned from searches, in a blinded manner. Full-texts were also reviewed blinded and in duplicate. Discrepancies at either phase were resolved by a third party. Multiple publications were collated to ensure we obtained the most detailed description of interventions (28).
Data collection process and data items
The following data items were tabulated in an excel database:
1. Administrative: title, author, acronym, number of papers, year, country, design (main), design (sub-type), sample details (arm name, sample size), potential for meta-analysis.
2. Sample details (mean and standard deviations): age, multi-morbidity data (mean, validated measures, disease counts).
3. Intervention details: description, categories based on description, non-exercise components, prescription, titration, location, duration, deliverer, underpinning research.
4. Outcomes: primary outcome, secondary outcome, follow-up schedule, follow-up time point used in analysis, raw outcome data, authors’ conclusions.
Risk of bias in individual studies
Risk of bias is not relevant to this descriptive review and will be reported with the planned meta-analysis.
Synthesis of results
Grouping, standardisation, synthesis methods, certainty
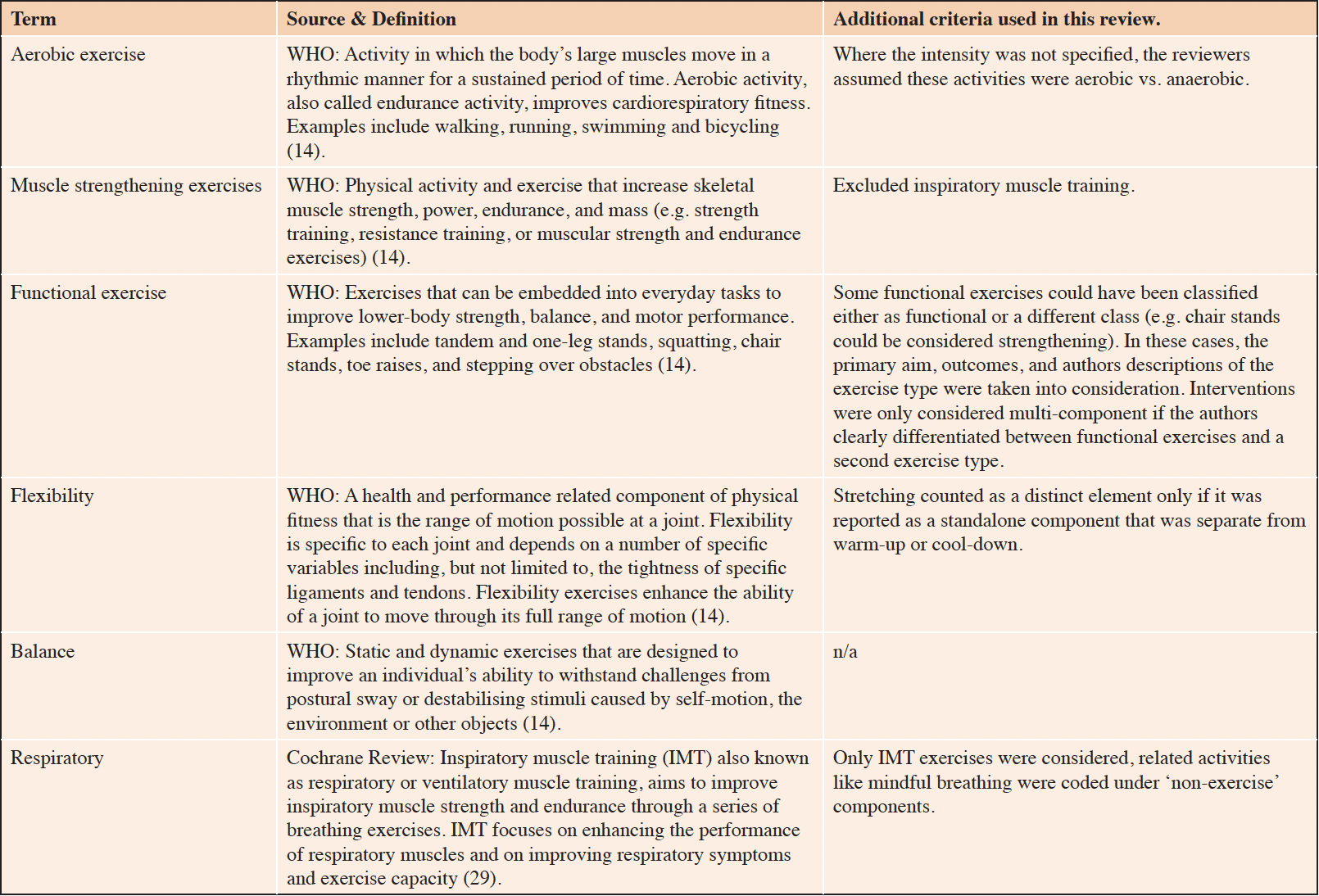
We grouped studies based on the number and type of exercise components they included on the supposition this would reveal the extent of heterogeneity within the category ‘multi-component’. To achieve standardisation, two blinded researchers reviewed all studies and coded the exercise intervention into six categories based on the description given in the paper or supporting texts. Exercise components were defined using WHO definitions (14) for aerobic physical activity, muscle strengthening exercises, flexibility exercise, balance exercises and functional exercises (Table 1). A further category for ‘respiratory exercises’ was used to include inspiratory muscle training. Exercises undertaken in different environments (e.g. hydrotherapy) were assessed and coded based on the components included.
In addition to characterising exercises included in interventions, we assessed the rationale behind intervention exercise content. Assessment was based on descriptions and references given within texts and categorised as either 1) based on pre-development work; 2) based on previous research studies; 3) based on national/international guidelines; 4) based on practitioner intuition/local practice. Studies that made no reference to either development work, previous research or guidelines were assumed to be based on ‘practitioner intuition/local practice’.
Results
Study selection
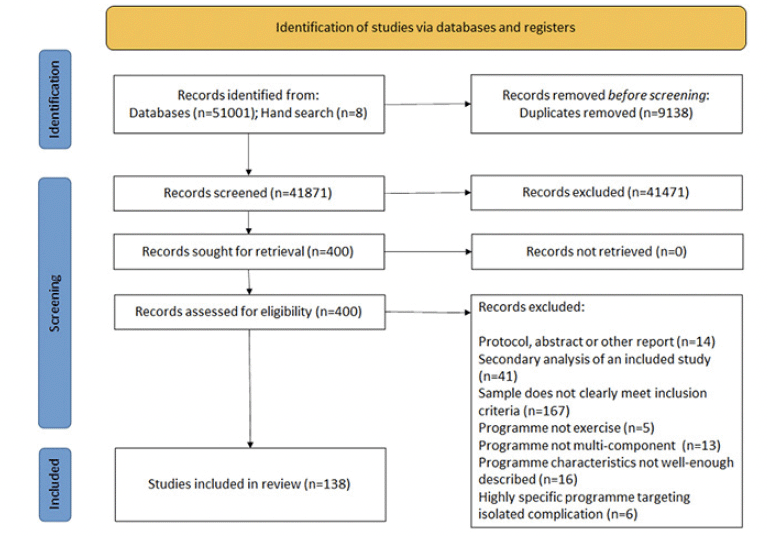
Seven databases were searched yielding a total of 51,001 papers, of these, 138 articles reporting unique interventions were included. Study references are provided in the supplementary material. Searches for associated texts to assess exercise types (e.g. reports of protocols, pilots and development work) resulted in a total of 259 articles.
Study characteristics
General characteristics
Characteristics of included studies are provided in Supplementary Tables 2 to 5. Studies were conducted from the period 1988 to 2020 and were predominantly undertaken in Europe and North America (75.3%). Almost half (44%) had another report associated with the study. Only 12 (8%) described themselves as multi-component and only four specifically stated they were targeting multi-morbidity. Most used randomised controlled designs (75.4%).
Participant characteristics
Across studies, 22,610 participants with a mean age of 72.1 years (range 60-92) were enrolled. Studies largely did not report mean number of comorbidities; combining statistics from those that did (n=36, 26%) gives a mean of 3.4 comorbidities (range: 2-6.6). Twelve reports described assessing multi-morbidity via validated measures. Most frequently used (n=9) was the Charlson Comorbidity Index (CCI);(30) however the actual score was only reported in seven studies (mean CCI: 3.39).
The majority of studies targeted either one, two or three chronic diseases (n=79, 57.2%; n=8, 5.8%; n=2, 1.4% respectively). Activity (n=20, 14.5%), risk factors (n=19, 13.8%), social care settings (n=7, 5.1%) or body composition (n=3, 2.2%) were other target criteria. By chronic disease, the most frequently represented were cardiovascular (n=24, 17.4%), followed by respiratory (n=14, 10.1%), cerebrovascular (n=11, 8%) and metabolic conditions (n=9, 6.5%). Physical function disorders were relatively regularly targeted (n=12, 8.7%) as were geriatric conditions (n=10, 7.2%) and frailty (n=10, 7,2%.) Only a limited number of comorbid conditions were counted by most studies and it is likely the burden of disease was significantly higher.
Exercise intervention characteristics
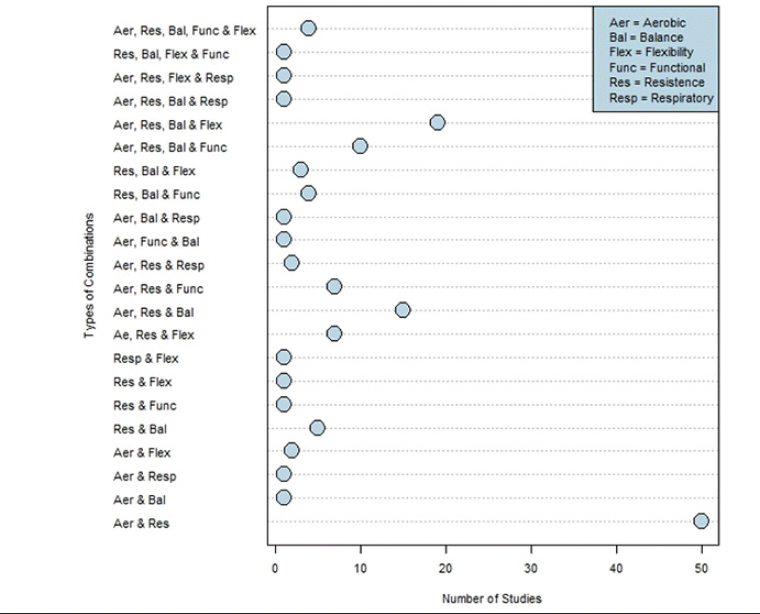
Overall, 22 different multi-component combinations were identified, see Figure 2. Few studies featured respiratory training (n=7, 5%). Seventy-three percent of studies were delivered twice per week and studies favoured a delivery duration of 60 minutes (n=51, 36%) for 12 weeks (n=24, 17%). Across programmes, the total exercise delivery time ranged from 4 to 430 hours with the average amount of exercise time prescribed being 180 minutes/week. Fifty-seven studies (41%) delivered interventions that did not meet recommended thresholds of 150 minutes a week (14).
Most interventions were centre based (n=78, 57%), followed by a combination of home and centre (n=29, 21%), exclusively home based (n=24, 17%) or delivered in a care home (n=7, 5%). Physiotherapists were the deliverer of choice across studies (n=66, 48%), followed by the multi-disciplinary team (MDT) (n=25, 18%) then exercise therapists (n=23, 17%). A group approach was favoured (n=106, 77%). Whilst this may indirectly provide socialisation benefits (31), few studies specifically described socialisation activities as part of the intervention (n=7, 5%). Group based studies generally require transportation, however only 11 studies gave details of transportation support; in most cases transportation arrangements were not clear (n=86); 11 studies were judged to have not provided transportation support.
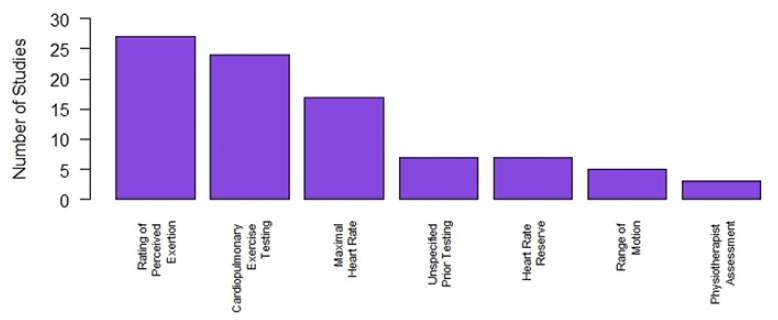
Descriptions of the formula for exercise intensity could be extracted from 92 of 138 studies (67%) (Figure 3). The most commonly used method to inform exercise intensity was the Borg rating of perceived exertion scale (RPE), followed by cardiopulmonary exercise testing and maximal heart rate (MHR). Based on studies reporting intensity by RPE or MHR, moderate intensity exercise was most commonly prescribed. Ninety-seven studies (70.2%) stipulated that the prescribed exercises were titrated, for the remaining programs (n=41, 29.7%) it was unclear whether exercise prescriptions were modified across the duration of the intervention.
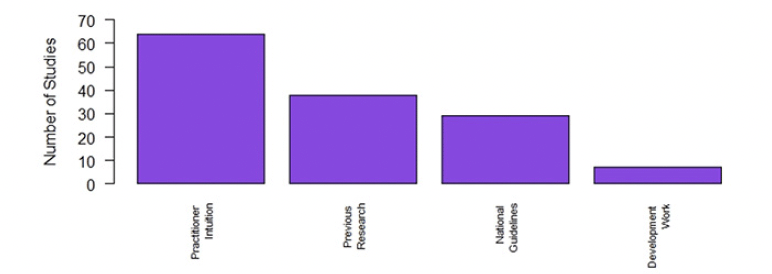
Choice of exercise type (Figure 4), frequency, intensity and duration were most frequently judged to be based on practitioner intuition/local practice (n=64, 46%). Of those citing a published study (n=38, 28%), twelve (35%) were based on four established programmes (Otago, Enhance Fitness, Sunbeam, High-Intensity Functional Exercise [HIFE]). For guideline-based studies (n=29, 21%), the most commonly referenced were recommendations from the American College of Sports Medicine that were contemporary to the research. Very few of the exercise programs were described as being based on developmental work (n=7, 5%); as is recommended for complex interventions (32, 33); like the programs described here.
Two-component studies
Studies largely favoured a 2-component exercise intervention (n=62, 45%) with the traditional combination of aerobic and resistance exercises being most common (n=49, 80.3%). Forty-four percent of 2-component studies included a non-exercise element, chiefly education (24.6%) or nutrition (8.2%). Most were centre based (n=37, 60.7%) or a combination of centre and home (n=13, 21%) and delivered by physiotherapists or exercise therapists (52.4%). Mean frequency was 3 times/week, giving an average exercise time of 152 minutes/week, for a duration of 138 days (range: 21-1095).
Three-component studies
The volume of studies reduced with each additional exercise type added. Forty-one studies (30%) included 3-component exercise interventions. The most common combination of exercise types was aerobic, resistance and balance (n=15, 36.6%.) A greater proportion (n=20, 49%) of 3-component studies were accompanied by non-exercise elements, principally education (61.9%), followed by behaviour change (14.3%) and psychological interventions (14.3%). Centre-based interventions were marginally more frequent (n=21, 51%) over a combined centre and home setting (48%). Physiotherapists were the intervention deliverer of choice (n=30, 73.1%), for a mean duration of 168 days (range 21-730). Average frequency was three times/week, giving a mean exercise time of 180 minutes/week.
Four-component studies
Thirty-one studies (22%) included a 4-component exercise intervention, these were dominated by aerobic, resistance, balance and flexibility combinations (n=19, 59.3%). Forty-five percent of 4-component interventions were accompanied by non-exercise elements (n=14), these were most often medical optimisation (23.8%) followed by education and nutrition (both n=4, 19%). The mean duration of intervention was 265 days (range: 28-1277 days) and physiotherapists were again the main deliverer (n=15, 46.8%), followed by education therapists (n=8, 25%) or the multi-disciplinary team (n=6, 18.7%). Exercises were prescribed on average three days per week for an average exercise time of 156 minutes/week.
Five-component studies
All 5-component studies (n=4, 3%) included the same combination of exercises: aerobic, resistance, balance, flexibility and functional. Only one of these was accompanied by a non-exercise component (medical optimisation). Mean duration was 130 days (range: 121-182 days) and three out of four interventions (75%) were delivered by the MDT. Mean frequency was seven times/week for an average of 275 minutes/week.
Discussion
General interpretation
This analysis found that multi-component exercise interventions targeting older adults with multi-morbidity include a great variety of combinations of exercise types (22 different combinations). The extent of this heterogeneity has significant implications for meta-analyses of effects. Grouping studies under the umbrella ‘multi-component’ could be problematic, as it is clear from this review that the interventions being grouped together can be manifestly different.
Many of the exercise types combined and the volume of exercise delivered by interventions, did not meet WHO recommendations for older adults with chronic conditions and/or mobility problems (14). Whilst we accept that many studies were not intentionally targeting either older adults nor multi-morbidity, they frequently targeted a chronic condition (64%) and all included both older adults with multi-morbidity. Lack of consideration of, and adaptation for, age-related and comorbid conditions within single-condition studies is concerning. Particularly as the types of chronic diseases or age related deficits which were the focus of many studies included in this review, are associated with multi-morbidity and guidance exists to support clinicians and researchers to do this (25, 34, 35).
A recent review of behavioural interventions reported that, from a sample of 600 studies, 68.3% excluded people with multiple chronic conditions (36). A larger study aimed at quantifying the exclusion of individuals with certain characteristics from 43,895 clinical trials listed in ClinicalTrials.gov, reported that 40.5% of adults ≥60 years and 91.1% of people with multi-morbidity were excluded (37). Within the present analysis, 67 studies were judged to be based on previous literature or guidelines that would have drawn upon research with similar stringent exclusion criteria. Therefore, it follows that researchers are extrapolating efficacy of interventions which may be effective in a single-condition, to people with that condition plus additional co-morbidity/multi-morbidity. Within settings where there is a long history of implementation and a wealth of research, like cardiac and pulmonary rehabilitation, there is evidence demonstrating the modifying effects of aging and multi-morbidity on efficacy (11, 38, 39). This has led many to call for a move away from ‘disease focussed’ to ‘person-focussed’ care (11).
The inclusion of multiple types of exercises within a single session necessitates consideration of the therapeutic dose being delivered. Whilst there are targets for overall activity (14), guidance is less clear regarding the amount of time that should be devoted to each component, particularly within a multi-component context (16). Across studies, these data could not be extracted due to insufficient description (only total time). However, with the exception of 5-component studies, there was no clear pattern of increased exercise time to accommodate additional components. Training time has been found to be an important modifier of effect in strength and aerobic training in older adults (40, 41).
Only seven studies (5%) included respiratory training. This number was lower than anticipated given the proportion of studies in the review that focussed on either cardiovascular or respiratory conditions (n=38, 27.5%). Within the categories cardiovascular or respiratory disease, chronic obstructive pulmonary disease and heart failure were the target diseases 36% and 54% of time respectively. Given that both diseases frequently result in symptoms of breathlessness; and considering the mounting evidence supporting the efficacy of this type of training in a broad range of cardiovascular (40) and respiratory diseases (41); the relative absence of this type of exercise was surprising. Breathlessness has been identified as a significant barrier to exercise in qualitative studies of older adults with multi-morbidity (43). Recognition of the potential wider role of respiratory training in older adults, out with respiratory disease, was evidenced in only one study in this review, which targeted falls (42).
Seventy-eight percent of studies were wholly or partly centre based, however very few studies described providing transportation support. Accessibility, proximity, convenience and transportation costs have also been identified as barriers to exercising for people with multi-morbidity (43, 44). Very few studies detailed socialisation type activities, either within the exercise intervention itself or as an adjunct. Reducing social isolation has been identified as an important priority for people with multi-morbidity (45). Both characteristics suggest that priorities that are important to people with multi-morbidity are not being considered when designing exercise interventions.
Exploration of the rationale behind selection of the exercise type may provide insight as to why studies that are including older adults with multi-morbidity are not making appropriate provisions when designing their intervention. Our assessment suggests that insufficient attention is directed towards selecting appropriate exercise type, with researchers often defaulting to generic guidelines or simply replicating previous ‘successful’ programmes.
Application of findings in clinical practice
Whilst these findings are more relevant to research practice, they do hold some clinical utility. Clinicians working with patients who experience multi-morbidity should be aware of the weaknesses in the evidence base identified by this review. Notably, that lack of consideration of the degree of heterogeneity within ‘multi-component’ designs and limiting testing of interventions that are cognisant of the barriers and or priorities of those with multi-morbidity. As guidelines synthesise current evidence, it follows that these weaknesses will be reflected within current recommendations.
Comparison with other reviews
Previous reviews (2, 3, 18, 19, 46, 47) have retrieved a far narrower pool of studies (range: 10-38) which we attribute to more exclusive search strategies, definitions of multi-morbidity or inclusion criteria. Most of these reviews have focussed on efficacy, therefore they do not offer a relevant comparison with the findings here. In their review of community based rehabilitation programmes for older adults with chronic conditions, Mulligan et al. (46) reported that most programmes focussed on education about disease management with few including ‘active’ exercise components; moreover, the choice of components was unclear.
Limitations of review process & evidence
Whilst this review has many strengths, notably its inclusive sampling in comparison to other reviews, there are some important limitations. Firstly, there is no universal definition of multi-morbidity and inclusion of different conditions will invariably enrich or dilute multi-morbidity prevalence (48). The broad criteria used here may have resulted in inclusion of studies without definitively multi-morbid samples. In an effort to keep the size of the review manageable, we excluded studies without a control group. Although these studies may have been relevant to the objectives of this review, given the number of studies included (n=138) we believe that their inclusion would be unlikely to meaningfully change our findings.
Much of the analysis was based on the presence or absence of certain information within a report. Therefore, if a study did not describe a particular criterion, it was noted as absent. As a result, some studies may have inadvertently been judged less favourably, purely as a result of journal imposed word counts and reporting standards. Lastly, studies published after the search date would not have been included. A re-run of our search in January 2024 identified four studies that would have met our inclusion criteria. A re-run of our search conducted in January 2024 in one of the databases, identified four studies that would have met our inclusion criteria.
Conclusions
This review has highlighted that multi-component exercise interventions that include older adults with multi-morbidity are highly heterogenous and therefore should not necessarily be grouped as an intervention sub-type within meta-analyses of effects. Future meta-analysis should carefully consider how best to combine multi-component exercise interventions, to ensure meaningful results are generated. Assessment of characteristics suggests that interventions are often not considerate of the abilities or needs of those with multi-morbidity, nor attuned to the participation barriers they experience. Such factors should be given greater consideration when designing future exercise interventions.
Acknowledgements: we would like to acknowledge the Patient and Public Involvement Group set up as part of the OPIMISE HFpEF study for suggesting and advising on this paper.
Funding: This systematic review was funded by the Evelyn Trust (personal award FF).
Data availability: data will be made available upon reasonable request.
Conflict of interests: Nil.
Authors’ Contributions: Faye Forsyth: Conception and design, acquisition of data, analysis and interpretation of data, drafted the article, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Jonathan Mant: Analysis and interpretation of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Christi Deaton: Conception and design, acquisition of data, analysis and interpretation of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Peter Hartley: Acquisition of data, analysis and interpretation of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Helen Lin: Acquisition of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Chien Lin Soh: Acquisition of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Kris Bailey: Acquisition of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Natasha Elks: Acquisition of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work. Scott Rowbotham: Analysis and interpretation of data, revised the draft critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work.
Data Availability: Data will be made available upon request.
Systematic Review registration number: CRD42020209672 accessible here: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=209672.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Di Lorito C, Long A, Byrne A, Harwood RH, Gladman JRF, Schneider S, et al. Exercise interventions for older adults: A systematic review of meta-analyses. J Sport Health Sci. 2021;10(1):29-47. DOI: 10.1016/j.jshs.2020.06.003
2. de Vries NM, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Staal JB, Nijhuis-van der Sanden MW. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: a meta-analysis. Ageing Research Reviews. 2012;11(1):136-49. DOI: 10.1016/j.arr.2011.11.002
3. Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205. DOI: 10.1136/bmj.e5205
4. Boehmer KR, Gionfriddo MR, Rodriguez-Gutierrez R, Dabrh AM, Leppin AL, Hargraves I, et al. Patient capacity and constraints in the experience of chronic disease: a qualitative systematic review and thematic synthesis. BMC Fam Pract. 2016;17:127. DOI: 10.1186/s12875-016-0525-9
5. Rosbach M, Andersen JS. Patient-experienced burden of treatment in patients with multimorbidity – A systematic review of qualitative data. PLoS One. 2017;12(6):e0179916. DOI: 10.1371/journal.pone.0179916
6. Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65(10):1041-51. DOI: 10.1016/j.jclinepi.2012.05.005
7. Moffat K, Mercer SW. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam Pract. 2015;16:129. DOI: 10.1186/s12875-015-0344-4
8. Willadsen TG, Siersma V, Nicolaisdóttir DR, Køster-Rasmussen R, Jarbøl DE, Reventlow S, et al. Multimorbidity and mortality: A 15-year longitudinal registry-based nationwide Danish population study. J Comorb. 2018;8(1):2235042×18804063. DOI: 10.1177/2235042X18804063
9. Jacunski M, Melville P, Currie GP. Exercise: the panacea in management of many ills. Now is the time to engage. J R Coll Physicians Edinb. 2021;51(2):120-2. DOI: 10.4997/JRCPE.2021.203
10. Devereux-Fitzgerald A, Powell R, Dewhurst A, French DP. The acceptability of physical activity interventions to older adults: A systematic review and meta-synthesis. Soc Sci Med. 2016;158:14-23. DOI: 10.1016/j.socscimed.2016.04.006
11. Holland AE, Harrison SL, Brooks D. Multimorbidity, frailty and chronic obstructive pulmonary disease: Are the challenges for pulmonary rehabilitation in the name? Chron Respir Dis. 2016;13(4):372-82. DOI: 10.1177/1479972316670104
12. Forman DE, Maurer MS, Boyd C, Brindis R, Salive ME, Horne FM, et al. Multimorbidity in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. 2018;71(19):2149-61. DOI: 10.1016/j.jacc.2018.03.022
13. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. British Journal of Sports Medicine. 2020;54(24):1451-62. DOI: 10.1136/bjsports-2020-102955
14. World Health Organisation. WHO guidelines on physical activity and sedentary behaviour. 2020. https://www.who.int/publications/i/item/9789240015128 (accessed 05/03/2024)
15. Valenzuela PL, Saco-Ledo G, Morales JS, Gallardo-Gómez D, Morales-Palomo F, López-Ortiz S, et al. Effects of physical exercise on physical function in older adults in residential care: a systematic review and network meta-analysis of randomised controlled trials. Lancet Healthy Longev. 2023;4(6):e247-e56. DOI: 10.1016/S2666-7568(23)00057-0
16. Baker MK, Atlantis E, Fiatarone Singh MA. Multi-modal exercise programs for older adults. Age Ageing. 2007;36(4):375-81. DOI: 10.1093/ageing/afm054
17. Barker K, Holland AE, Skinner EH, Lee AL. Clinical Outcomes Following Exercise Rehabilitation in People with Multimorbidity: A Systematic Review. J Rehabil Med. 2023;55:jrm00377.
18. Bricca A, Harris LK, Jager M, Smith SM, Juhl CB, Skou ST. Benefits and harms of exercise therapy in people with multimorbidity: A systematic review and meta-analysis of randomised controlled trials. Ageing Res Rev. 2020;63:101166. DOI: 10.1016/j.arr.2020.101166
19. Bricca A, Jager M, Johnston M, Zangger G, Harris LK, Midtgaard J, et al. Effect of In-Person Delivered Behavioural Interventions in People with Multimorbidity: Systematic Review and Meta-analysis. Int J Behav Med. 2023;30(2):167-89. DOI: 10.1007/s12529-022-10092-8
20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. 2022. https://training.cochrane.org/handbook
21. Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. DOI: 10.1136/bmj.l6890
22. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. DOI: 10.1136/bmj.b2535
23. World Health Organisation. Multimorbidity: Technical Series on Safer Primary Care. 2016. https://www.who.int/publications/i/item/9789241511650 (accessed 05/03/2024)
24. Johnston MC, Crilly M, Black C, Prescott GJ, Mercer SW. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. 2019;29(1):182-9. DOI: 10.1093/eurpub/cky098
25. Chowdhury SR, Chandra Das D, Sunna TC, Beyene J, Hossain A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine. 2023;57:101860. DOI: 10.1016/j.eclinm.2023.101860
26. Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(1):245. DOI: 10.1186/s13643-017-0644-y
27. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. DOI: 10.1186/s13643-016-0384-4
28. Lefebvre C GJ, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, Noel-Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Chapter 4: Searching for and selecting studies. . In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) Cochrane Handbook for Systematic Reviews of Interventions version 62 (updated February 2021) Cochrane, 2021. https://training.cochrane.org/handbook/current/chapter-04 (accessed 05/03/2024)
29. Ammous O, Feki W, Lotfi T, Khamis AM, Gosselink R, Rebai A, et al. Inspiratory muscle training, with or without concomitant pulmonary rehabilitation, for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2023;1(1):CD013778. DOI: 10.1002/14651858.CD013778.pub2
30. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83. DOI: 10.1016/0021-9681(87)90171-8
31. Pels F, Kleinert J. Loneliness and physical activity: A systematic review. International Review of Sport and Exercise Psychology. 2016;9(1):231-60. 10.1080/1750984X.2016.1177849
32. Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. DOI: 10.1136/bmj.n2061
33. O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9(8):e029954. DOI: 10.1136/bmjopen-2019-029954
34. Dekker J, Buurman BM, van der Leeden M. Exercise in people with comorbidity or multimorbidity. Health Psychol. 2019;38(9):822-30. DOI: 10.1037/hea0000750
35. Dekker J, de Rooij M, van der Leeden M. Exercise and comorbidity: the i3-S strategy for developing comorbidity-related adaptations to exercise therapy. Disabil Rehabil. 2016;38(9):905-9. DOI: 10.3109/09638288.2015.1066451
36. Stoll CRT, Izadi S, Fowler S, Philpott-Streiff S, Green P, Suls J, et al. Multimorbidity in randomized controlled trials of behavioral interventions: A systematic review. Health Psychol. 2019;38(9):831-9. DOI: 10.1037/hea0000726
37. Tan YY, Papez V, Chang WH, Mueller SH, Denaxas S, Lai AG. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3(10):e674-e89. DOI: 10.1016/S2666-7568(22)00186-6
38. Listerman J, Bittner V, Sanderson BK, Brown TM. Cardiac rehabilitation outcomes: impact of comorbidities and age. J Mol Signal. 2011;31(6):342-8. DOI: 10.1097/HCR.0b013e31822f189c
39. Taylor JL, Medina-Inojosa JR, Chacin-Suarez A, Smith JR, Squires RW, Thomas RJ, et al. Age-Related Differences for Cardiorespiratory Fitness Improvement in Patients Undergoing Cardiac Rehabilitation. Front Cardiovasc Med. 2022;9:872757. DOI: 10.3389/fcvm.2022.872757
40. Borde R, Hortobágyi T, Granacher U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015;45(12):1693-720. DOI: 10.1007/s40279-015-0385-9
41. Visser D, Wattel EM, Gerrits KHL, Wouden JCvd, Meiland FJM, Groot AJd, et al. Effectiveness and characteristics of physical fitness training on aerobic fitness in vulnerable older adults: an umbrella review of systematic reviews. BMJ Open. 2022;12(5):e058056. DOI: 10.1136/bmjopen-2021-058056
42. Zak M, Gryglewska B. Application of two structured rehabilitation regimens in the frail octogenarians (over 85) with functional disorders. Rehabilitacja medyczna. 2006;10(2):20-4. https://bibliotekanauki.pl/articles/1935838 (accessed 05/03/2024)
43. Jager M, Lindhardt MC, Pedersen JR, Dideriksen M, Nyberg M, Bricca A, et al. Putting the pieces together: A qualitative study exploring perspectives on self-management and exercise behavior among people living with multimorbidity, healthcare professionals, relatives, and patient advocates. J Multimorb Comorb. 2022;12:26335565221100172. DOI: 10.1177/26335565221100172
44. Falck RS, Davis JC, Milosevic E, Liu-Ambrose T. How much will older adults exercise? A feasibility study of aerobic training combined with resistance training. Pilot Feasibility Stud. 2017;3:2. DOI: 10.1186/s40814-016-0116-5
45. Parker SG, Corner L, Laing K, Nestor G, Craig D, Collerton J, et al. Priorities for research in multiple conditions in later life (multi-morbidity): findings from a James Lind Alliance Priority Setting Partnership. Age and Ageing. 2019;48(3):401-6. DOI: 10.1093/ageing/afz014
46. Mulligan H, Wilkinson A, Chen D, Nijhof C, Kwan N, Lindup A, et al. Components of community rehabilitation programme for adults with chronic conditions: A systematic review. International Journal of Nursing Studies. 2019;97:114-29. DOI: 10.1016/j.ijnurstu.2019.05.013
47. Barker K, Holland AE, Lee AL, Haines T, Ritchie K, Boote C, et al. Multimorbidity rehabilitation versus disease-specific rehabilitation in people with chronic diseases: a pilot randomized controlled trial. Pilot feasibility stud. 2018;4(1):181. DOI: 10.1186/s40814-018-0369-2
48. Ho IS, Azcoaga-Lorenzo A, Akbari A, Davies J, Hodgins P, Khunti K, et al. Variation in the estimated prevalence of multimorbidity: systematic review and meta-analysis of 193 international studies. BMJ Open. 2022;12(4):e057017. DOI: 10.1136/bmjopen-2021-057017
The Author(s) 2024