P. de Souto Barreto1,2, M. Cesari3,4, J.E. Morley5, E. Gonzalez-Bautista1,2, Y. Rolland1,2, D. Azzolino4, B. Vellas1,2,6, R.A. Fielding7
Task force members: Sandrine Andrieu (Toulouse, France); Mylène Aubertin Leheudre (Montreal, Canada); Nuria Barcons (Barcelona, Spain); Ann Beliën (Heusden-Zolder, Belgium); Carla Delannoy (Vevey, Switzerland); Groarke John (New York, USA); Luis Miguel Gutierrez Robledo (Mexico City Mexico); Darren Hwee (South San Francisco, USA); Nathan LeBrasseur (Rochester, USA); Jean Mariani (Paris, France); Merchant Reshma (Singapour, Singapore); Suzette Pereira (Columbus, USA); Quann Erin (Vevey, Switzerland); Rossulek Michelle (New York, USA); Ricardo Rueda (Columbus, USA); Sandrine Sourdet (Toulouse, France); Lisa Tarasenko (New York, USA); Cendrine Tourette (Paris, France); Rob Van Maanen (Paris, France); Debra L. Waters (Dunedin, New Zealand, Albuquerque, New Mexico); Heather Whitson (Durham, USA)
1. Gérontopôle de Toulouse, Institut du Vieillissement, Centre Hospitalier-Universitaire de Toulouse, Toulouse, France; 2. CERPOP, Inserm 1295, Université de Toulouse, UPS, Toulouse, France; 3. IRCCS Istituti Clinici Scientifici Maugeri, University of Milan, Milano, Italy; 4. Department of Clinical and Community Sciences, University of Milan, Milan, Italy; 5. Division of Geriatric St Louis University Medical School, St Louis, USA; 6. UNM Division of General Internal Medicine and Geriatric Medicine, University of New Mexico, School of Medicine, USA; 7. Nutrition, Exercise Physiology, and Sarcopenia Laboratory, Jean Mayer USDA, Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA
Corresponding Author: Philipe de Souto Barreto, Gérontopôle de Toulouse, Institut du Vieillissement, 37 Allées Jules Guesde, 31000 Toulouse, France, +33 561 145 636, Electronic address: philipebarreto81@yahoo.com.br
J Frailty Aging 2023;12(1)1-6
Published online December 21, 2022, http://dx.doi.org/10.14283/jfa.2022.64
Abstract
The Appetite loss in older people is an important unmet clinical need in geriatrics. The International Conference on Frailty and Sarcopenia Research (ICFSR) organized a Task Force on April 20th 2022, in Boston, to discuss issues related to appetite loss in older people, in particular, the assessment tools currently available, its evaluation in the primary care setting, and considerations about its management. There is a high heterogeneity in terms of the etiology of appetite loss in older people and a gold standard assessment tool for evaluating this condition is still absent. Although this may render difficult the management of poor appetite in clinical practice, validated assessment tools are currently available to facilitate early identification of appetite loss and support care decisions. As research on biomarkers of appetite loss progresses, assessment tools will soon be used jointly with biomarkers for more accurate diagnosis and prognosis. In addition, efforts to foster the development of drugs with a favorable risk/benefit ratio to combat poor appetite should be strengthened.
Key words: Appetite loss, anorexia of aging, nutritional status, older adults, intrinsic capacity, ICOPE.
Introduction
Appetite loss is increasingly recognized as a significant clinical problem among older adults. Its high prevalence (1-4) and association with adverse health events (including weight loss and malnutrition, mood-related conditions, sarcopenia, and frailty (1, 5-9)), demonstrate the central role poor appetite may play in older adults’ health. Although loss of appetite, alongside weight loss, is a flagship hallmark of malnutrition during aging, its assessment and management in clinical settings are not performed systematically in geriatric populations..
Recognizing appetite loss as a major unmet clinical need in older people, the International Conference on Frailty and Sarcopenia Research (ICFSR) organized the first Task Force in September 2021 to raise awareness around this topic (10). Following up on this prior effort, the ICFSR Task Force met again on 20th April 2022 to debate appetite loss in older people, in particular, appetite assessment tools, its evaluation in the primary care setting, and considerations about its management. The present article reports on the main outputs from this ICFSR Task Force held in Boston in 2022.
Appetite and aging: assessment tools
Self-reported tools of appetite
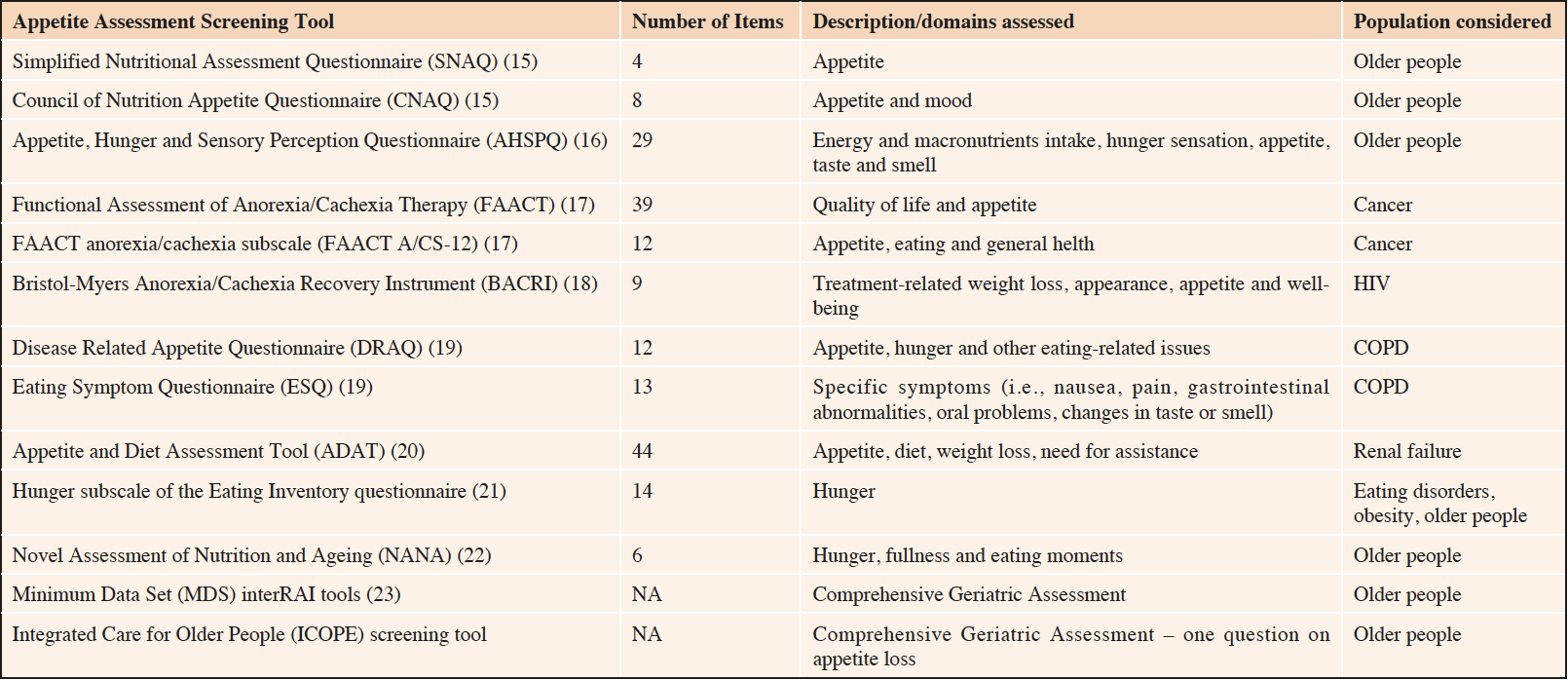
Appetite in older adults can be assessed through subjective ratings. Given the subjective nature of lack of appetite as well as the multifactorial causes underlying the condition, appetite assessment presents some challenging issues. Several tools to screen for malnutrition in older people (i.e., Mini Nutritional Assessment (11), Birmingham Nutrition Risk Score (12), Malnutrition Screening Tool (13), Short Nutrition Assessment Questionnaire (14)) include appetite loss as a component, recognizing its importance as a determinant of malnutrition. However, these tools aim to identify malnutrition, and the loss of appetite may, therefore, remain unrecognized in individuals who present with this complaint in isolation. Several screening tools have been developed and validated to identify older people with poor appetite or at risk of developing it (Table 1).
Likert and visual analogue scales (VAS) are among the most commonly used tools to screen for appetite decline in older people. The Simplified Nutritional Assessment Questionnaire (SNAQ) (15), is a simple screening tool composed of 4 items. Each item is scored from 1 to 5 on a Likert scale. A score lower than 14 indicates an increased risk of weight loss (i.e., at least 5%) within six months. The SNAQ showed a good predictive ability for weight loss and undernutrition (15, 24). The SNAQ was developed as a short version of the Council of Nutrition Appetite Questionnaire (CNAQ), which consists of 8 items on a 5-point Likert scale, with lower scores indicating deterioration in appetite (15). In particular, possible CNAQ scores range from 8 (worst) to 40 (best). A total CNAQ score ≤28 indicates a significant risk of at least 5% weight loss within six months. Both the SNAQ and the CNAQ were derived from the Appetite, Hunger and Sensory Perception Questionnaire (AHSPQ) (16). The AHSPQ was developed for people aged 70 and older and includes 29 items focusing on the self-assessment of energy and macronutrients intake, hunger sensation, appetite, taste and smell. However, it is burdensome and time-consuming to complete in the clinical setting (25, 26). Hays and Roberts (27) suggested that the hunger subscale of the Eating Inventory questionnaire (21) may help identify older people at risk for anorexia of aging and unintentional weight loss. The hunger subscale includes 14 questions and has been reported to predict unintentional weight loss (28).
Brief instruments have been developed to reduce the burden that long and complex questionnaires may have on frail individuals. This is the case of the simplified anorexia questionnaire (29), which is constituted of only two items: the first item assesses the lack of appetite on a scale ranging from 0 to 10 and the second item evaluates the severity of lack of appetite during the past week. The simplified anorexia questionnaire showed a moderate correlation with the 12-item Functional Assessment of Anorexia/Cachexia Therapy (FAACT) anorexia/cachexia subscale (A/CS) (FAACT A/CS-12) when a single point in time was considered. However, this instrument lacked sensitivity over time and was poor in predicting survival in advanced cancer patients.
Instruments, such as the FAACT (17), have been developed to measure quality of life and appetite in disease-specific conditions (ie, cancer, HIV). The original version of the FAACT is composed of 39 items on a 5-point Likert scale. Given its length, the scoring system, and multidomain nature, the FAACT questionnaire is not easily implementable in the routine clinical practice (15). However, shorter versions, like the FAACT A/CS-12, have been developed and validated (17, 30). Similarly, the Bristol-Myers Anorexia/Cachexia Recovery Instrument (BACRI) (18), a 9-item visual analogue instrument, was developed to assess treatment-related changes on anorexia/cachexia in HIV patients. However, its implementation out of the context of an interventional trial is challenging (17). Other tools to assess anorexia/appetite loss in specific patient populations include the Disease Related Appetite Questionnaire (DRAQ) and the Eating Symptom Questionnaire (ESQ), which have been developed for chronic obstructive pulmonary disease patients (19). The DRAQ is a 12-item questionnaire on a 5-level scale derived from the CNAQ; it evaluates appetite, hunger and other eating-related issues. The ESQ is constituted of 13 items on a 5-point Likert scale about symptoms such as nausea, pain, gastrointestinal tract abnormalities, oral problems, and changes in taste or smell in the past two weeks that could all be clearly related to the feelings of hunger and appetite. In addition, the ESQ includes an open item in which additional symptoms can be reported. The appetite and diet assessment tool (ADAT) was developed to evaluate appetite as well as other factors affecting dietary intake in hemodialysis patients (20). The ADAT is a 44-item, self-administered questionnaire regarding appetite, recent changes in dry weight, dietary compliance, need for assistance with food shopping and meal preparation, common food practices, and the patient’s perceptions of food enjoyment and diet satisfaction.
In recent years, computerized instruments to measure appetite in the older population have been developed. Brown et al. (22), validated a 6-item computerized tool for measuring older adults’ self-reported mood and appetite as part of the Novel Assessment of Nutrition and Ageing (NANA) toolkit. In particular, appetite was evaluated using six visual analogue scale items (ie, hunger, fullness, desire to eat, perception of amount they could eat, urge to eat, or preoccupation with thoughts of food), with a 11-point (ranging from 0 [not at all] to 10 [very]) Likert scale response. The implementation of digital tools to assess even remotely appetite may be of great importance, especially in times of pandemic or for those individuals that cannot attend outpatient clinics. Furthermore, remote assessment of appetite would facilitate the implementation of repeated measurements, what would provide a more accurate picture of this symptom since repeated assessments would better capture fluctuations in appetite.
Landi et al. (31) suggested the use of comprehensive geriatric assessment tools like the Minimum Data Set (MDS) interRAI tools (23). In particular, the interRAI tools include a set of multidimensional geriatric instruments aimed at identifying clinical, psycho-social and environmental problems in older persons in order to implement a personalized intervention. Finally, appetite loss is also screened by single questions; such an approach has been recently operationalized in the screening tool used in the context of the World Health Organization (WHO) program of Integrated Care for Older People (ICOPE) for evaluating the vitality domain (screen for malnutrition) of intrinsic capacity (IC). Besides vitality, the ICOPE screening tool also explores for potential deficits in other functional domains: locomotion, cognition, vision, hearing, and psychology.
In sum, there are a multitude of assessment tools already available for evaluating loss of appetite in older people, without a consensual gold standard. The degree of scientific validation varies across tools; some only apply to specific clinical populations.
Biomarkers for assessing appetite
Appetite status can also be measured through the levels of specific biomarkers of satiation and satiety (32), but such laboratory markers are mainly used for research purposes; they are not easily implementable in the routine clinical practice. Ghrelin, the so-called “hunger hormone”, which is synthesized and released in the stomach, seems to act both in the short term (ie, meal initiation) and in the long term (ie, after weight loss) (32). However, ghrelin levels seem not to change during aging (33). Leptin has been related to long-term appetite and acts as a satiety signal (34). In particular, leptin regulates food ingestion in conditions of energy imbalance (32). Other peptides potentially involved in the pathophysiology of appetite loss include cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1), whose increased levels are associated with lower feelings of hunger and lower food intake, playing a role in meal termination (32,35). In addition, some reports have outlined the role of peptide YY (PYY) as a marker of satiety. Of note, augmented concentrations of PYY during the postprandial period have been observed in older people suggesting a role of this peptide in determining less desire for a second meal (31). Altered insulin regulation, which is frequently observed in older people, has been suggested to play a role in appetite probably through its effects on leptin and ghrelin regulation (31). However, given its involvement in several metabolic processes, insulin’s role as a satiety marker is less clear (32). More recently, the growth differentiation factor 15 (GDF15), a cytokine involved in stress-signaling in several physiological processes (36), has been recognized as a putative appetite regulator (37), being associated to appetite-related adverse events (eg, anorexia, cachexia) in both animal (38,39) and human (40) investigations. Interestingly, GDF15 is related with increased chronological and biological age (41), mitochondrial dysfunction (42), and inflammatory status (43). This suggests GDF15 could play an important role in different aging-dependent processes, including poor appetite and food intake. Further investigations on the longitudinal associations of GDF15 and appetite loss during aging in different age-ranges are welcome.
Although biomarkers of poor appetite constitute a promising way for early risk stratification of future loss of appetite, its utility in clinical settings remains to be proven. Research advances rendering currently expensive techniques more accessible in the future, particularly in terms of unbiased investigations (eg, OMICS approach), may lead to important discoveries in this field in the coming years.
Appetite loss in primary care: the Toulouse implementation of the WHO ICOPE program
The WHO ICOPE program represents an innovative healthcare pathway promoting functional ability and healthy aging, particularly in older adulthood. It is focused on the improvement/maintenance of optimal levels of IC, the composite of an individual’s physical and mental capacities, operationalized using six domains: locomotion, cognition, psychology, vision, hearing, and vitality (the domain most closely related to nutritional status) (44–46). The screening of the vitality domain is performed using questions on appetite loss and unintentional weight loss.
The ICOPE program has been implemented in the real-life healthcare system of the French, South West region Occitanie (Toulouse area), since January 2020 (47). More than 20,000 individuals have already been screened, in particular by primary care providers (48), using the ICOPE screening tool. All the data is collected and stored in a secured database (48). We describe below (Table 2) results of this ICOPE implementation experience using data abstracted at the end of February 2022, regarding individuals aged 60 years or older (mean age 76.7 years ±8.8; 62% women), assessed by healthcare professionals and with information on appetite loss.
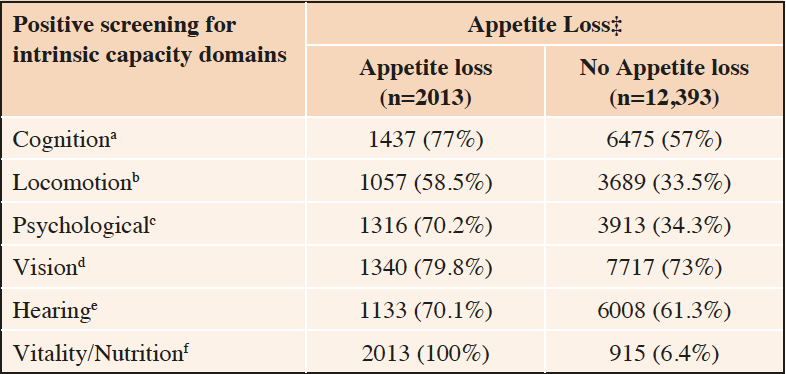
Table 2. Positive screening for the domains of intrinsic capacity according to appetite loss status using the ICOPE screening tool† in older adults in the French region Occitanie – data abstracted on February 2022*
*Population varied due to missing data; ‡Percentages reflect the prevalence of positive screenings in intrinsic capacity within the groups with and without appetite loss; †Operationalization of the ICOPE screening tool is described elsewhere(44). Operational definitions are summarized below (letters a to f); a. Cognition (n=13,255): wrong response to 3-word recall or orientation items; b. Locomotion (n=12,814): Unable to complete five chair rises in ≤ 14 seconds; c. Psychological (n=13,280): self-reporting to feel down, depressed or hopeless OR having little interest or pleasure in doing things in the past two weeks; d. Vision (n=12,253): self-reporting to have eyes problems (difficulties in seeing far, reading, eye diseases or currently under medical treatment (eg, diabetes, high blood pressure)); e. Hearing (n=11,411): Hearing impairment as measured through the whisper test; f. Vitality (n=14,406): screening for malnutrition based on self-report of any of unintentional weight loss and appetite loss
Data from this ICOPE implementation experience showed that, among the 14,358 individuals with data on both appetite and weight loss, 1,995 subjects had appetite loss and 1,778 had weight loss. Only 43.2% (n=863) of those reporting to have appetite loss had a concomitant unintentional weight loss; this represents 48.5% of people with weight loss who also had appetite loss. Compared to those without this condition, people with appetite loss had an increased probability (p-value < 0.05 for all) of having positive screenings in all the other domains of IC, even when the cross-sectional binary logistic regressions were adjusted by age, sex, and body weight. Such cross-sectional associations were particularly robust for locomotion, cognition, and psychology; they remained significant even when disentangling the effects of appetite loss from those of weight loss (ie, when comparing people with appetite loss without weight loss to people with none of those conditions). Similar adjusted cross-sectional associations of appetite loss were found among people (sample varying from 419 to 801 individuals according to the domain of IC) receiving an in-depth assessment of locomotion (short physical performance battery, SPPB (49)), psychology (Patient Health Questionnaire depression module, PHQ-9 (50)), cognition (Mini-Mental State Examination, MMSE (51)), and as expected nutritional status (Mini-nutritional assessment, MNA (11)). Furthermore, individuals with appetite loss, compared to those without appetite loss, had higher severity of frailty (Fried frailty phenotype (52)) and sarcopenia (SARC-F (53)), lower handgrip strength, and worst performance in basic and instrumental activities of daily living (sample size varying across measurements from 587 to 1,238 people).
Among people without baseline positive screening for specific domains of IC, longitudinal (mean follow-up length of 6.5 ± 2.1 months) binary logistic regressions adjusted by age, sex and body weight showed that having appetite loss at baseline was associated with the incidence of positive screenings (using the ICOPE screening tool) on cognition (n=1649; OR 1.83, 95% CI 1.20 – 2.80, p=0.005), locomotion (n=985; OR 1.62, 95% CI 1.00 – 2.61, p=0.05), and psychology (n=2527; OR 1.73, 95% CI 1.08 – 2.76, p=0.02). Appetite loss was associated with the incidence of weight loss (n=3515; OR 1.78, 95% CI 1.13 – 2.80, p=0.013).
Regarding longitudinal data on appetite loss, 4,106 individuals assessed by care professionals provided this information at least twice, 6.5 ±2.1 months apart. Among the 459 individuals with appetite loss at baseline, 98 (21.4%) still had appetite loss at follow-up (persistent appetite loss), whereas 361 (78.6%) no longer had this condition (appetite loss reversibility). In this short time, the incidence of appetite loss among the 3,647 individuals without this condition at baseline was only 4.6% (n=167).
Appetite loss management: a few considerations
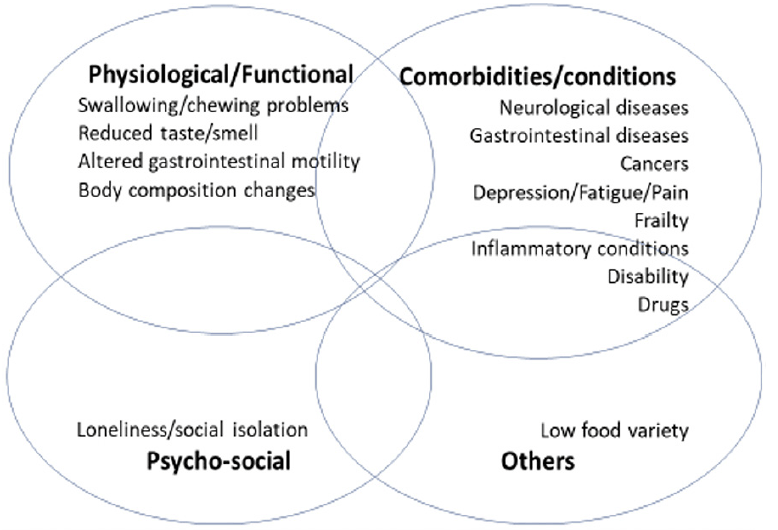
Since no drug therapies are approved for the indication of poor/loss of appetite currently, non-pharmacological approaches remain the frontline treatment for this condition. Given the multifactorial nature of loss of appetite during aging (see Figure 1 on the determinants of appetite loss adapted from De Souto Barreto et al. (10)), a multidimensional approach to this condition is recommended. In the same vein, multimodal intervention strategies may be needed according to the potential causes of poor appetite. Therefore, a comprehensive geriatric assessment and the consequent implementation of multi-domain interventions should be considered and prioritized.
Appetite loss may represent a warning for future weight loss and malnutrition (54). It is associated with a myriad of adverse health events, even when other overt clinical signs (eg, weight loss) of malnutrition are absent. Among potential strategies to treat appetite loss and its consequences, we can cite flavor enhancements (eg, spices, herbs, masking bitter taste (27, 55)), food fortification, mineral/vitamins and oral nutritional supplements (31, 56), but also physical exercise (10). Modifications of food texture, palatability and presentation, increased dietary variety, use of finger foods, and the provision of feeding assistance may be helpful (31). Adapting the social environment (31, 57) related to food intake, such as the time dedicated to meals, surroundings quiet and calm, promoting conviviality and a positive atmosphere, and having well-lighted places may contribute to improving appetite in older adults.
Even though the multifactorial etiology of appetite loss impacts on the possibility to have a straightforward/unique drug indication, research on pharmacological interventions for treating this condition should be strengthened. Examples from modern medicine of pharmacologically treatable multidimensional symptomatology exist and can be illustrated by pain, for example. Like appetite loss, pain has several determinants (eg, physiological, anatomical, psycho-social, comorbidities and chronic diseases) and this condition can be relieved by symptomatic treatments. Such an example should constitute a goal to be pursued by researchers in the field of appetite and anorexia of aging.
Final considerations
Appetite loss in older people is a critical unmet clinical need in geriatrics. Unfortunately, the high heterogeneity in terms of the etiology of appetite loss in older people and the absence of a gold standard assessment tool for evaluating this symptom render its management in clinical practice difficult. Despite those pitfalls, validated assessment tools exist. These tools are mostly used for research purposes, with no appetite assessment tool systematically used in clinical practice currently. However, these instruments may already be used to identify appetite loss as early as possible, before more pronounced signs of malnutrition (eg, weight loss) arise. Given its simplicity, easiness-of-use, and robust associations with malnutrition, the SNAQ instrument may constitute a good assessment tool for implementation in clinical settings. For a broad, public health approach, single-item measurements, such as the vitality item operationalized in the WHO ICOPE program, should be prioritized to screening for possible appetite loss.
Future research on this area should focus on investigating the phenotyping of people with appetite loss more accurately since different clinical profiles (eg, appetite loss with/without: weight loss; depression; mobility impairment; cognitive decline) probably require different management strategies. Furthermore, efforts are needed for improving the understanding of the natural history of appetite loss (eg, longitudinal evolution according to age-ranges and sex, incidence, persistence, reversibility) in older people and the feasibility of using appetite assessment tools in different real-life clinical care settings. The implementation of a biorepository in those future studies would foster biased (eg, GDF15) and unbiased investigations on markers and targets of appetite loss during aging.
As research progresses, in the near future, such assessment tools will probably be used jointly with biomarkers of appetite loss for achieving a more accurate diagnosis. In parallel, the scientific community should support the efforts toward developing pharmacological approaches to combat poor appetite.
Acknowledgments: Dr Fielding’s participation was supported by the U.S. Department of Agriculture (USDA), under agreement No. 58-1950-4-003; any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Conflict of Interests: The Task Force was partially funded by registration fees from industrial participants. These corporations placed no restrictions on this work. Dr. de Souto Barreto reports other from Pfizer, outside the submitted work; Dr. Cesari reports personal fees from Nestlé Health Science, outside the submitted work; Dr. Morley has nothing to disclose; Dr. Gonzalez-Bautista has nothing to disclose; Dr. Rolland has nothing to disclose; Dr. Azzolino has nothing to disclose; Dr Vellas is an investigator in clinical trials sponsored by the Toulouse University Hospital (Inspire Geroscience Program). Rejuvenate Biomed Clinical Advisory Board Meeting, Pfizer research grant; Dr. Fielding reports grants from National Insitutes of Health, grants from USDA Agricultural Research Service, grants, personal fees and other from Axcella Health, other from Juvicell, other from Inside Tracker, grants and personal fees from Biophytis, personal fees from Amazentis, personal fees from Nestle, personal fees from Pfizer, outside the submitted work.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Malafarina V, Uriz-Otano F, Gil-Guerrero L, Iniesta R. The anorexia of ageing: physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas. 2013 Apr;74(4):293–302.
2. Cox NJ, Ibrahim K, Sayer AA, Robinson SM, Roberts HC. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients. 2019 Jan 11;11(1):E144.
3. Landi F, Picca A, Calvani R, Marzetti E. Anorexia of Aging: Assessment and Management. Clin Geriatr Med. 2017 Aug;33(3):315–23.
4. Roy M, Gaudreau P, Payette H. A scoping review of anorexia of aging correlates and their relevance to population health interventions. Appetite. 2016 Oct 1;105:688–99.
5. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Barillaro C, et al. Association of anorexia with sarcopenia in a community-dwelling elderly population: results from the ilSIRENTE study. Eur J Nutr. 2013 Apr;52(3):1261–8.
6. Morley JE. Anorexia of ageing: a key component in the pathogenesis of both sarcopenia and cachexia. J Cachexia Sarcopenia Muscle. 2017 Aug;8(4):523–6.
7. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients. 2016 Jan 27;8(2):69.
8. Sanford AM. Anorexia of aging and its role for frailty. Curr Opin Clin Nutr Metab Care. 2017 Jan;20(1):54–60.
9. Martone AM, Onder G, Vetrano DL, Ortolani E, Tosato M, Marzetti E, et al. Anorexia of aging: a modifiable risk factor for frailty. Nutrients. 2013 Oct 14;5(10):4126–33.
10. de Souto Barreto P, Cesari M, Morley JE, Roberts S, Landi F, Cederholm T, et al. Appetite Loss and Anorexia of Aging in Clinical Care: An ICFSR Task Force Report. J Frailty Aging. 2022;11(2):129–34.
11. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999 Feb 1;15(2):116–22.
12. Reilly HM, Martineau JK, Moran A, Kennedy H. Nutritional screening – Evaluation and implementation of a simple Nutrition Risk Score. Clin Nutr. 1995 Oct 1;14(5):269–73.
13. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999 Jun 1;15(6):458–64.
14. Kruizenga HM, Van Tulder MW, Seidell JC, Thijs A, Ader HJ, Van Bokhorst-de van der Schueren MAE. Effectiveness and cost-effectiveness of early screening and treatment of malnourished patients. Am J Clin Nutr. 2005 Nov;82(5):1082–9.
15. Wilson MMG, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005 Nov;82(5):1074–81.
16. de Jong N, Mulder I, de Graaf C, van Staveren WA. Impaired sensory functioning in elders: the relation with its potential determinants and nutritional intake. J Gerontol A Biol Sci Med Sci. 1999 Aug;54(8):B324-331.
17. Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, et al. Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2000;9(10):1137–46.
18. Cella DF, VonRoenn J, Lloyd S, Browder HP. The Bristol-Myers Anorexia/Cachexia Recovery Instrument (BACRI): a brief assessment of patients’ subjective response to treatment for anorexia/cachexia. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 1995 Jun;4(3):221–31.
19. Nordén J, Grönberg AM, Bosaeus I, Forslund HB, Hulthén L, Rothenberg E, et al. Nutrition impact symptoms and body composition in patients with COPD. Eur J Clin Nutr. 2015 Feb;69(2):256–61.
20. Burrowes JD, Powers SN, Cockram DB, McLeroy SL, Dwyer JT, Cunniff PJ, et al. Use of an appetite and diet assessment tool in the pilot phase of a hemodialysis clinical trial: Mortality and morbidity in hemodialysis study. J Ren Nutr. 1996 Oct 1;6(4):229–32.
21. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83.
22. Brown LJE, Adlam T, Hwang F, Khadra H, Maclean LM, Rudd B, et al. Computerized Self-Administered Measures of Mood and Appetite for Older Adults: The Novel Assessment of Nutrition and Ageing Toolkit. J Appl Gerontol Off J South Gerontol Soc. 2018 Feb;37(2):157–76.
23. Bernabei R, Landi F, Onder G, Liperoti R, Gambassi G. Multidimensional Geriatric Assessment: Back to the Future Second and Third Generation Assessment Instruments: The Birth of Standardization in Geriatric Care. J Gerontol Ser A. 2008 Mar 1;63(3):308–13.
24. Rolland Y, Perrin A, Gardette V, Filhol N, Vellas B. Screening older people at risk of malnutrition or malnourished using the Simplified Nutritional Appetite Questionnaire (SNAQ): a comparison with the Mini-Nutritional Assessment (MNA) tool. J Am Med Dir Assoc. 2012 Jan;13(1):31–4.
25. Andreae C, Strömberg A, Sawatzky R, Årestedt K. Psychometric Evaluation of Two Appetite Questionnaires in Patients With Heart Failure. J Card Fail. 2015 Dec 1;21(12):954–8.
26. Hanisah R, Suzana S, Lee FS. Validation of screening tools to assess appetite among geriatric patients. J Nutr Health Aging. 2012 Jul;16(7):660–5.
27. Hays NP, Roberts SB. The anorexia of aging in humans. Physiol Behav. 2006 Jun 30;88(3):257–66.
28. Hays NP, Bathalon GP, Roubenoff R, McCrory MA, Roberts SB. Eating Behavior and Weight Change in Healthy Postmenopausal Women: Results of a 4-Year Longitudinal Study. J Gerontol Ser A. 2006 Jun 1;61(6):608–15.
29. Davis MP, Yavuzsen T, Kirkova J, Walsh D, Karafa M, LeGrand S, et al. Validation of a Simplified Anorexia Questionnaire. J Pain Symptom Manage. 2009 Nov 1;38(5):691–7.
30. Homsi J, Walsh D, Rivera N, Rybicki LA, Nelson KA, Legrand SB, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2006 May;14(5):444–53.
31. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, et al. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients. 2016 Jan 27;8(2):69.
32. de Graaf C, Blom WAM, Smeets PAM, Stafleu A, Hendriks HFJ. Biomarkers of satiation and satiety. Am J Clin Nutr. 2004 Jun;79(6):946–61.
33. Morley JE. Anorexia, weight loss, and frailty. J Am Med Dir Assoc. 2010 May;11(4):225–8.
34. Yeung AY, Tadi P. Physiology, Obesity Neurohormonal Appetite And Satiety Control. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 Feb 10]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK555906/
35. Cox NJ, Morrison L, Ibrahim K, Robinson SM, Sayer AA, Roberts HC. New horizons in appetite and the anorexia of ageing. Age Ageing. 2020 Jul 1;49(4):526–34.
36. Adela R, Banerjee SK. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J Diabetes Res. 2015;2015:1–14.
37. Mullican SE, Rangwala SM. Uniting GDF15 and GFRAL: Therapeutic Opportunities in Obesity and Beyond. Trends Endocrinol Metab. 2018 Aug 1;29(8):560–70.
38. Johnen H, Lin S, Kuffner T, Brown DA, Tsai VWW, Bauskin AR, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007 Nov;13(11):1333–40.
39. Borner T, Shaulson ED, Ghidewon MY, Barnett AB, Horn CC, Doyle RP, et al. GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 2020 Feb 4;31(2):351-362.e5.
40. Tsai VWW, Husaini Y, Manandhar R, Lee-Ng KKM, Zhang HP, Harriott K, et al. Anorexia/cachexia of chronic diseases: a role for the TGF-β family cytokine MIC-1/GDF15. J Cachexia Sarcopenia Muscle. 2012;3(4):239–43.
41. Liu H, Huang Y, Lyu Y, Dai W, Tong Y, Li Y. GDF15 as a biomarker of ageing. Exp Gerontol. 2021 Apr;146:111228.
42. Poulsen NS, Madsen KL, Hornsyld TM, Eisum ASV, Fornander F, Buch AE, et al. Growth and differentiation factor 15 as a biomarker for mitochondrial myopathy. Mitochondrion. 2020 Jan;50:35–41.
43. Tavenier J, Rasmussen LJH, Andersen AL, Houlind MB, Langkilde A, Andersen O, et al. Association of GDF15 With Inflammation and Physical Function During Aging and Recovery After Acute Hospitalization: A Longitudinal Study of Older Patients and Age-Matched Controls. J Gerontol A Biol Sci Med Sci. 2021 May 22;76(6):964–74.
44. World Health Organization. Integrated care for older people (ICOPE): Guidance for person-centred assessment and pathways in primary care. 2019.
45. WHO | WHO Guidelines on Integrated Care for Older People (ICOPE) [Internet]. WHO. [cited 2019 Jan 25]. Available from: http://www.who.int/ageing/publications/guidelines-icope/en/
46. World Health Organization. World report on ageing and health. [Internet]. WHO Press; 2015. Available from: https://www.who.int/ageing/events/world-report-2015-launch/en/
47. Tavassoli N, de Souto Barreto P, Berbon C, Mathieu C, de Kerimel J, Lafont C, et al. Implementation of the WHO integrated care for older people (ICOPE) programme in clinical practice: a prospective study. Lancet Healthy Longev. 2022 Jun 1;3(6):e394–404.
48. Tavassoli N, Piau A, Berbon C, De Kerimel J, Lafont C, De Souto Barreto P, et al. Framework implementation of the inspire icope-care program in collaboration with the world health organization (who) in the occitania region. J Frailty Aging 2021;10(2)103-109;http://dx.doi.org/10.14283/jfa.2020.26
49. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85-94.
50. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13.
51. Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–98.
52. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146-156.
53. Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013 Aug;14(8):531–2.
54. Rolland Y, Perrin A, Gardette V, Filhol N, Vellas B. Screening older people at risk of malnutrition or malnourished using the Simplified Nutritional Appetite Questionnaire (SNAQ): a comparison with the Mini-Nutritional Assessment (MNA) tool. J Am Med Dir Assoc. 2012 Jan;13(1):31–4.
55. Hamerman D. Molecular-Based Therapeutic Approaches in Treatment of Anorexia of Aging and Cancer Cachexia. J Gerontol Ser A. 2002 Aug 1;57(8):M511–8.
56. Cox NJ, Ibrahim K, Sayer AA, Robinson SM, Roberts HC. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients. 2019 Jan 11;11(1):E144.
57. Pilgrim AL, Robinson SM, Sayer AA, Roberts HC. An overview of appetite decline in older people. Nurs Older People. 2015 Jun;27(5):29–35.
© The Author(s) 2022