M. PAHOR1, S.B. KRITCHEVSKY2, D.L. WATERS3, D.T. VILLAREAL4, J. MORLEY5, J.M. HARE6, B. VELLAS7,8,9 AND THE ICFSR TASK FORCE
1. University of Florida Institute on Aging, Gainesville, FL, USA; 2. Sticht Center for Healthy Aging and Alzheimer’s Prevention. Wake Forest School of Medicine. Winston-Salem, NC USA; 3. University of Otago, Dunedin School of Medicine, Dunedin, New Zealand; 4. Baylor College of Medicine and Michael E DeBakey VA Medical Center, Houston, TX, USA; 5. Division of Geriatrics, St. Louis, University Medical School, St. Louis, MO, USA; 6. Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, Miami, FL, USA; 7. UMR1027 Inserm, F-31073, Toulouse, France; 8. University of Toulouse III, F-31073, France; 9. Gérontopôle Toulouse, Toulouse University Hospital, F-31000, Toulouse, France;
Corresponding author: Marco Pahor, University of Florida Institute on Aging, Gainesville, FL, USA, mpahor@ufl.edu
Task Force members: Hidenori Arai (Obu City, Japan); Mylène Aubertin (Montréal, Canada); Jürgen Bauer (Heidelberg, Germany); Ryne Carney (Washington, USA); Brian Clark (Athens, USA); Alfonso Cruz Jentoft (Madrid, Spain); Carla Delannoy (Vevey, Switzerland); Susanna Del Signore (Paris, France); Elsa Dent (Adelaide, Australia); Waly Dioh (Paris, France); Roger Fielding (Boston, USA); Bertrand Fougère (St Louis, USA); Juerg Gasser (Basel, Switzerland); Jack Guralnik (Baltimore, USA); Hare Joshua (Miami, USA); Aaron Hinken (King of Prussia, USA); Evgueni Ivanov (Basel, Switzerland); Naotoshi Kanemitsu (Tokyo, Japan); Kala Kaspar (Vevey, Switzerland); Tatiana Klompenhouwer (Utrecht, The Netherlands); Stephen Kritchevsky (Winston-Salem, USA); Francesco Landi (Roma, Italy); Valérie Legrand (Nanterre, France); Yvette Luiking (Utrecht, The Netherlands); Ram Miller (Cambridge, USA); Bradley Morgan (South San Francisco, USA); John Morley (St Louis, USA); Vikkie Mustad (Columbus, USA); David Neil (King of Prussia, USA); Suzanne Page (Miami, USA); Marco Pahor (Gainesville, USA); Dimitris Papanicolaou (East Hanover, USA); Suzette Pereira (Columbus, USA); Claire Regard (Vevey, Switzerland); Daniel Rooks (Cambridge, USA); Jorge Ruiz (Miami, USA); Cornel Sieber (Nürnberg, Germany); Sitra Tauscher Wisniewski (Northbrook, USA); Brooke Travnicek (Clearwater, USA); Vellas Bruno (Toulouse, France); Dennis Villareal (Houston, USA); Debra Waters (Dunedin,New Zealand); Lixin Zhang Auberson (Basel, Switzerland)
J Frailty Aging 2018;7(3):150-154
Published online July 9, 2018, http://dx.doi.org/10.14283/jfa.2018.20
Abstract
To reduce disability and dependence in older adults, frailty may represent an appropriate target for intervention. While preventing frailty through lifestyle interventions may be the optimal public health approach for many population groups, pharmacological approaches will likely be needed for individuals who meet frailty criteria or who have comorbid conditions that contribute to and complicate the frailty syndrome, and for those who are not compliant with lifestyle interventions. Barriers to successful development of drug treatments for frailty include variability in how the frailty syndrome is defined, lack of agreement on the best diagnostic tools and outcome measures, and the paucity of sensitive, reliable, and validated biomarkers. The International Conference on Frailty and Sarcopenia Research Task Force met in Miami, Florida, on February 28, 2018, to consider the status of treatments under development for frailty and discuss potential strategies for advancing the field. They concluded that at the present time, there may be a more productive regulatory pathway for adjuvant treatments or trials targeting specific functional outcomes such as gait speed. They also expressed optimism that several studies currently underway may provide the insight needed to advance drug development for frailty.
Key words: Sarcopenia, frailty, gait speed, short physical performance battery, clinical trials.
Introduction
The frailty syndrome has emerged as a public health priority worldwide (1) and a major contributor to late-life disability, loss of independence (2), poor health outcomes, and increased costs (3). Consensus definitions of frailty conceptualize the condition as progressive functional decline and increased vulnerability to stress resulting from decreased physiological reserve and resilience (1, 4, 5). To reduce disability and dependence in older adults, frailty may thus represent an appropriate target for intervention (6). Complex genetic, physiologic, and psychosocial processes contribute to the development of frailty (7). As a result, both lifestyle and pharmacological approaches will likely be required to successfully prevent and treat frailty. Recognizing the need to accelerate development of treatments for frailty, the International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force met in February 2018 to address the challenges faced in designing clinical trials to assess the efficacy of these interventions in older populations.
Non-pharmacologic approaches to preventing frailty
Evidence supports the use of exercise (8), nutritional support (9, 10), and reduction of polypharmacy as efficacious approaches to the treatment of frailty (11, 12). Poor nutrition is a major risk factor for frailty (13), and nutritional approaches such as the Mediterranean diet (14) or supplementation with specific nutrients such as Vitamin D (15) or the leucine metabolite beta-hydroxy-beta-methylbutyrate (HMB) (16) have also been shown to improve physical performance in older adults. Polypharmacy, or the use of more than five drugs simultaneously, has also been associated with increased risk of frailty (17), and the combination of frailty and polypharmacy is associated with poorer outcomes, including increased mortality (18, 19). Reducing overprescription of drugs via medication optimization may thus represent a beneficial strategy for combatting frailty (20).
One of the most important studies in recent years has been the Lifestyle Interventions and Independence for Elders (LIFE) study, which showed that among a group of sedentary adults at risk for mobility disability, a physical activity (PA) intervention, despite being relatively modest in terms of intensity and duration, was superior to health education alone in preventing major and persistent mobility disability as measured using the 400-meter walk test (21). The study was notable for its sample size and strong study design. Subgroup analysis showed older and those with lower Short Physical Performance Battery (SPPB) scores benefited most from the intervention. In the LIFE pilot study, PA was also shown to be more effective than health education in reducing the frailty burden, especially in those with frailty or comorbidity at baseline (22). Further analysis of the LIFE data indicated that results differed depending on the outcome measure used: while the PA intervention was associated with improvement in frailty measured with the Fried criteria (23), it did not alter other measures of frailty (24).
Challenges for drug trials
While preventing frailty through lifestyle interventions may be the optimal public health approach for many population groups (25), pharmacological approaches will likely be needed for individuals who meet frailty criteria or who have comorbid conditions that contribute to and complicate the frailty syndrome (26). In order to conduct clinical trials for drugs targeting frailty, however, several challenges must first be addressed. First, to ensure more consistent diagnosis and more reliable selection of appropriate trial participants, alignment is needed between those who view frailty as vulnerability or risk (23, 27) and those who see it as a multidimensional continuum (28). While these two perspectives open the door to different approaches, the use of two different definitions also leads to variability in the selection of trial parameters such as inclusion criteria and outcome measures.
In selecting a target population at risk of frailty, many factors need to be considered, including age, low physical activity, impaired physical function, impaired cognition, disability (activities of daily living [ADLs] and instrumental ADLs [IADLs]), comorbidities, involuntary weight loss, lack of social support, incontinence, depression, exhaustion and fatigue, history of hospitalization, polypharmacy, sensory deficits, low-grade inflammation, pressure sores risk, and of course, clinical judgement. In the LIFE study, the physical activity intervention was most effective in the most frail group (24), indicating that this high risk group is an excellent target for interventions to reduce major mobility disability. Many frailty assessment instruments have been proposed for different purposes (e.g., to assess risk of adverse outcomes, to assess risk factors for clinical studies, or for clinical decision making.). Frailty has only infrequently been used as an outcome in interventional studies. A systematic study of frailty instruments identified 67, of which nine were most frequently cited (29). Among the nine highly-cited instruments all assess physical function but only six include assessment of disability, three assess physical activity, four assess cognition, five assess comorbidity, two assess weight loss, and five assess other factors such as social, sensory, or demographic (29).
Selecting primary and secondary outcomes represents another challenge for investigators designing trials. As mentioned earlier, the selection of outcome measure can determine whether a study succeeds or fails (24). Nonetheless, both the LIFE and Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) studies have demonstrated that it is feasible to assess outcomes in intervention trials for frailty (30).
A related trial, called the ENabling Reduction of low-Grade Inflammation in Seniors (ENRGISE) pilot study is now underway to examine whether improvements in mobility can also be achieved by reducing the level of inflammatory markers with a nutritional supplement (fish oil) and the angiotensin receptor blocker losartan (31). Primary outcome measures in this study include changes in interleukin-6 (IL-6) levels and changes in the 400-meter walk test. Secondary outcome measures include the SPPB, frailty according to the Fried/CHS criteria (23), other measures of muscle strength and power, and a patient-reported measure of disability. This study represents a transition to show if the outcomes demonstrated in non-pharmacological trials can be reproduced in studies with drugs and nutraceuticals. Supporting this approach, a cross-sectional analysis of data from the Women’s Health and Aging Studies (WHAS) demonstrated correlation between markers of inflammation and the prevalence of frailty in community-dwelling older women (32).
In selecting outcome measures for clinical studies, investigators must balance the desire to better understand mechanistic pathways and responses to treatment with participant burden and controlling the overall cost of the study.
Selecting drugs or other interventions to be tested in frailty trials represents another challenge. The choice of treatments should be based on a solid understanding of pathophysiology of frailty, which is complex and not fully understood. It may be necessary to treat frailty using a multimodal approach tailored for individual patients.
Progress in testing drugs for frailty – human mesenchymal stem cells
Mesenchymal stem cells (MSCs) derived from bone marrow have been shown to have potent anti-inflammatory, anti-fibrotic, neoangiogenic, and pro-regenerative properties that may have therapeutic potential for many diseases of aging, including frailty (33). Hare and colleagues have shown that hMSCs can be delivered safely, circulate throughout the body, localize to areas of inflammation, and retain effectiveness in older individuals (33, 34). In collaboration with Longeveron, they launched a clinical trial program in 2014 called the AllogeneiC Human Mesenchymal Stem Cells in Patients with Aging, FRAilTy via intravenoUS Delivery (CRATUS) Project (NCT02065245) to establish the safety of allogeneic human MSCs in individuals with frailty, determine the efficacy parameters in various domains of functional capacity and quality of life, and evaluate the usefulness of biomarkers to assess clinical responses in individuals with aging frailty
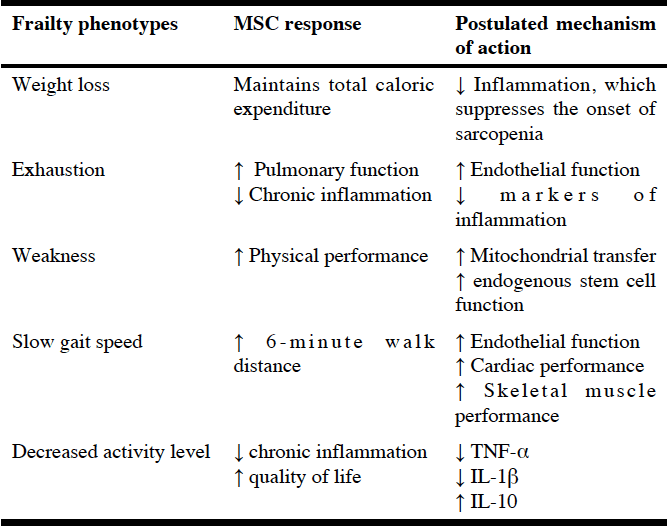
In the initial Phase I non-blinded study, 15 participants aged 60-95 who met frailty criteria established by the Canadian Study on Health and Aging were given escalating doses by intravenous infusions of 20, 100, or 200 million allo-hMSCs (35). A second, Phase I/II study enrolled 30 participants randomized to receive placebo or either 100 million or 200 million allo-hMSCs. The primary outcome measure for safety was the incidence of treatment-related serious adverse effects such as death, pulmonary embolism, stroke, worsening dyspnea resulting in hospitalization, or clinically significant laboratory tests abnormalities. Secondary efficacy endpoints included reduced rate of decline as measured by the 4-meter gait speed test and the 6-minute walk test (6MWT); weight loss; decreased handgrip strength assessed by dynamometer and SPPB; exhaustion assessed using the multidimensional fatigue inventory questionnaire; difference in quality of life assessment; death from any cause; exercise change in ejection fraction; and a panel of inflammatory biomarkers (36).
TNF-α – Tumor Necrosis Factor alpha; IL-1β – interleukin-1 beta; IL-10 – interleukin 10; Note: MSCs home to sites of injury and enhance repair of damaged tissues (heart, joints, muscle, blood vessels) and exert their regenerative effects via paracrine signaling, mitochondrial transfer, direct cellular contact, and exosome excretion.
The infusions were well tolerated, with no treatment-related serious adverse events. Blood tests at baseline and at 6 and 12 months after the infusions showed a dose-related reduction in markers of inflammation, notably marked and sustained declines in TNF-α, as well as a decreased number of “exhausted” B cells, suggesting improved immunosenescence. The results in terms of frailty measures are shown in Table 1 (37).
A Phase IIb dose-ranging multicenter clinical trial (n=120) is now underway with a more narrowly defined target population, i.e., a clinical frailty scale score of 5-6 and 6MWT between 200 and 400 meters, as well as a Tumor Necrosis Factor alpha (TNFα) level ≥ 2.5 pg/ml. The primary outcome in this trial will be a change in the 6MWT. Secondary outcomes will include change in TNFα level and score on the PROMIS Physical Function Patient Reported Outcome assessment. Exploratory endpoints will include other physical performance measures, a frailty score, upper and lower extremity function patient-report outcome (PRO) scores, falls efficacy scale score, spirometry, neuroinflammatory biomarkers, Performance Oriented Mobility Assessment, and clinical outcomes. The study is powered to show a difference in the 6MWT between 3 different treatment groups (dose-response) and placebo groups at 6 months. Thirty (30) subjects per treatment arm will provide 80% power to demonstrate an effect size of 0.75, defined as the treatment difference of each dose vs. placebo in change from baseline in 6MWT divided by the common standard deviation, at α=0.05.
Biomarkers in drug trials
Biomarkers are essential for treatment development yet have received little attention since relatively few studies have been conducted to treat frailty. In the context of clinical trials, biomarkers can provide mechanistic insight or serve as intermediate or surrogate endpoints. An ideal trial biomarker for frailty should 1) be associated with frailty independent of age and comorbidities, predict what frailty predicts (i.e., disability), and be on the causal pathway to the target outcome; 2) be sensitive to change in response to interventions that affect the risk or severity of frailty: rapidly responding biomarkers allow for shorter trials and 3) be insensitive to common treatments used in older populations, not overly burdensome, and show low within-subject variation.
Composite biomarkers that combine several measurements into a single summary scale have shown promise in epidemiological studies but may include measures not targeted in a clinical trial, or measures that are not sensitive enough to detect change during the period of the trial. For example, Sanders and colleagues developed a modified physiologic index score combining measures of systolic blood pressure, forced vital capacity (FVC), the Digit-Symbol Substitution Test (DSST), serum cystatin C, and serum fasting glucose (38). This index was associated with incident disability and death, but it might not be useful in the clinical trial context. DSST and FVC may lack sufficient sensitivity to detect change over a 6- or 12-month study. Also, many older people have undiagnosed hypertension or glucose abnormalities that when detected by the study could lead to treatment which would add noise to study results. The interpretation of some physiologic measures varies over the life course, as low blood pressure is associated with lower mortality at younger ages, but higher mortality in the oldest old (39)complicating the interpretation of an index, which included blood pressure.
In 2013, López-Otin and colleagues described nine biological hallmarks of aging and proposed that health can be improved by directly targeting those hallmarks (40). Three hallmarks – inflammation, mitochondrial energetics, and senescence – contribute to frailty as demonstrated by their association with the five dimensions of the Fried/CHS frailty scale — weakness, slowness, low energy, weight loss, and inactivity. Many putative biomarkers of inflammation have been identified. In a study exploring the association of these biomarkers with physical function, Hsu and colleagues found that eight different biomarkers coalesced into independent TNF-α and C-reactive protein (CRP)-related factors. Both were associated with poor function but differed in their association with body composition (41).
Biomarkers will likely find most use for risk prediction and mechanistic insight and there are many candidate biomarkers. Repositories of tissue and serum from well-characterized people in the context of interventions that did or did not work will be essential to identify additional biomarkers and correlate them with phenotypes. The strongest candidates identified thus far are related to inflammation. IL-6 and TNF-α soluble receptor (either 1 or 2), and possibly TNF-α itself. Other multi-dimensional biomarker panels including T- and B-cell subsets are presently being evaluated in the CRATUS trial cohort. Both are associated with weakness and muscle loss, yet it is as yet unclear whether these associations are specific to or a companion to inflammation and whether they are in the causal pathway of frailty. Trials targeting inflammation may provide answers to this question.
Conclusions
A regulatory pathway for frailty interventions would require a better understanding of the biological pathways that contribute to frailty and a clearer definition of frailty as an outcome. It was suggested that the next step for the ICFSR Task Force might be to convene a consensus conference to define frailty. Alternatively, lacking a consensus definition, it may be more productive to develop adjuvant treatments rather than targeting frailty itself.
Some Task Force members suggested that frailty may be too heterogeneous to be used as an intervention target, and that functional measures such as gait speed, chair rise, or stair climb performance are more reasonable outcomes to target. Given the heterogeneity of individuals with frailty, it would also be helpful to define subgroups that can be tested with different interventions to see how they respond. For example, frailty may be associated with obesity, malnutrition, etc., and more research is needed to understand how these other factors may lead to frailty. Biomarker profiles could enable individualized approaches to treatment but will only become possible with multi-marker strategies and complex statistical methodology.
The Fried criteria are the most widely used to define frailty and to classify individuals as frail or prefrail (23). However, it may be necessary to define different stages of frailty itself, e.g. mild, moderate and severe. In addition, the Fried criteria focus only on physical frailty, yet there are also social, cognitive (42), and psychological forms of frailty. The Rockwood approach captures additional elements to define frailty but may be somewhat onerous for clinicians and patients to administer (28).
Intermediate endpoints and biomarkers are also needed for efficient clinical trials. Much more research is needed on biomarkers before the field can select and reach consensus on the most useful biomarkers. There is a regulatory pathway for qualification of biomarkers, but this will require a great deal more data than are currently available. While individual biomarkers may provide some mechanistic insight that enables the design of a successful trial, eventually it may be desirable to profile patients based on multiple factors including function, inflammatory markers, etc., in order to select the most appropriate treatment. However, at present there is insufficient knowledge about why some individuals respond to a treatment and others do not. Outcomes in the ENRGISE pilot study may provide some clarity on the relationship of inflammatory markers to treatment response, which may be especially important since most diseases of aging are linked to inflammation. ENRGISE is asking a simple question – is inflammation a bystander to frailty or is it in a pathway that can be modified through intervention. Only when that question has been answered will it make sense to move to additional questions, including what mechanisms and pathways are involved and what are the subsets of responders.
The SPRINTT investigators have proposed inability to complete the 400-meter walk as the primary endpoint, although European Medicines Association (EMA) approval of this endpoint is still pending. EMA would like to see additional data on the clinical relevance of failing the 400-meter walk test. Gait speed has been suggested as a more clinically relevant indicator.
Conflicts of interest: Marco Pahor: ENRGISE is funded by the National Institutes of Health grant number U01AG050499. Abbott provided a grant for study drug, but the company has no other involvement with the study. Joshua M. Hare: holds a patent for cardiac cell-based therapy and holds equity in Vestion Inc. He maintains a professional relationship with Vestion Inc. as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion Inc. did not play a role in the design, conduct, or funding of the study. Dr. Hare is the Chief Scientific Officer, a compensated consultant and advisory board member for Longeveron and holds equity in Longeveron. Dr. Hare is also the co-inventor of intellectual property licensed to Longeveron. Longeveron did not play a role in the design, conduct, or funding of the study.
Acknowledgements: The authors thank Lisa Bain for assistance in preparing this manuscript.
References
1. Cesari M, Prince M, Thiyagarajan JA, De Carvalho IA, Bernabei R, Chan P, et al. Frailty: An Emerging Public Health Priority. J Am Med Dir Assoc. 2016;17(3):188-92.
2. World Health Organization. World Report on Ageing and Health. Luxembourg; 2015.
3. Le Cossec C, Perrine AL, Beltzer N, Fuhrman C, Carcaillon-Bentata L. Pre-Frailty, Frailty, and Multimorbidity: Prevalences and Associated Characteristics from Two French National Surveys. J Nutr Health Aging. 2016;20(8):860-9.
4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-62.
5. Rodriguez-Manas L, Feart C, Mann G, Vina J, Chatterji S, Chodzko-Zajko W, et al. Searching for an Operational Definition of Frailty: A Delphi Method Based Consensus Statement. The Frailty Operative Definition-Consensus Conference Project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62-7.
6. Vellas B, Cestac P, Moley JE. Implementing frailty into clinical practice: we cannot wait. J Nutr Health Aging. 2012;16(7):599-600.
7. Vina J, Tarazona-Santabalbina FJ, Perez-Ros P, Martinez-Arnau FM, Borras C, Olaso-Gonzalez G, et al. Biology of frailty: Modulation of ageing genes and its importance to prevent age-associated loss of function. Mol Aspects Med. 2016;50:88-108.
8. Silva RB, Aldoradin-Cabeza H, Eslick GD, Phu S, Duque G. The Effect of Physical Exercise on Frail Older Persons: A Systematic Review. J Frailty Aging. 2017;6(2):91-6.
9. Guigoz Y. Frailty and Nutrition: What We Have Learned from Research and Clinical Practice on the Mini Nutritional Assessment. J Frailty Aging. 2012;1(2):52-5.
10. Goisser S, Guyonnet S, Volkert D. The Role of Nutrition in Frailty: An Overview. J Frailty Aging. 2016;5(2):74-7.
11. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-7.
12. Fielding RA, Travison TG, Kirn DR, Koochek A, Reid KF, von Berens A, et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: Results from the VIVE2 randomized trial. J Nutr Health Aging. 2017;in press.
13. Bonnefoy M, Berrut G, Lesourd B, Ferry M, Gilbert T, Guerin O, et al. Frailty and nutrition: searching for evidence. J Nutr Health Aging. 2015;19(3):250-7.
14. Fougere B, Mazzuco S, Spagnolo P, Guyonnet S, Vellas B, Cesari M, et al. Association between the Mediterranean-style Dietary Pattern Score and Physical Performance: Results from TRELONG Study. J Nutr Health Aging. 2016;20(4):415-9.
15. Lopez J, Campa A, Lewis JE, Huffman FG, Liuzzi JP, Li T, et al. Assessing the relationship between vitamin D status and impairments in cognitive and physical performance in older adults using a dual task physical performance test. J Prev Alz Dis. 2017;4(1):29-36.
16. Fitschen PJ, Wilson GJ, Wilson JM, Wilund KR. Efficacy of beta-hydroxy-beta-methylbutyrate supplementation in elderly and clinical populations. Nutrition. 2013;29(1):29-36.
17. Herr M, Robine JM, Pinot J, Arvieu JJ, Ankri J. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf. 2015;24(6):637-46.
18. Gutierrez-Valencia M, Izquierdo M, Cesari M, Casas-Herrero A, Inzitari M, Martinez-Velilla N. The relationship between frailty and polypharmacy in older people: A systematic review. Br J Clin Pharmacol. 2018.
19. Rosted E, Schultz M, Sanders S. Frailty and polypharmacy in elderly patients are associated with a high readmission risk. Dan Med J. 2016;63(9).
20. Muscedere J, Kim P, Aitken P, Gaucher M, Osborn R, Farrell B, et al. Proceedings of the Canadian Frailty Network Summit: Medication Optimization for Frail Older Canadians, Toronto, Monday April 24, 2017. Can Geriatr J. 2017;20(4):253-63.
21. Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387-96.
22. Cesari M, Vellas B, Hsu FC, Newman AB, Doss H, King AC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70(2):216-22.
23. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56.
24. Trombetti A, Hars M, Hsu FC, Reid KF, Church TS, Gill TM, et al. Effect of Physical Activity on Frailty: Secondary Analysis of a Randomized Controlled Trial. Annals of internal medicine. 2018;168(5):309-16.
25. Vellas B, Sourdet S. Prevention of Frailty in Aging. J Frailty Aging. 2017;6(4):174-7.
26. Cesari M, Fielding R, Benichou O, Bernabei R, Bhasin S, Guralnik JM, et al. Pharmacological Interventions in Frailty and Sarcopenia: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2015;4(3):114-20.
27. Bergman H, Hogan D, Karunananthan S. Frailty: A clinically relevant concept? Canadian J of Geriatrics. 2008;11(3):124-8.
28. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17-26.
29. Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61.
30. Marzetti E, Calvani R, Landi F, Hoogendijk EO, Fougere B, Vellas B, et al. Innovative Medicines Initiative: The SPRINTT Project. J Frailty Aging. 2015;4(4):207-8.
31. Manini TM, Anton SD, Beavers DP, Cauley JA, Espeland MA, Fielding RA, et al. ENabling Reduction of Low-grade Inflammation in SEniors Pilot Study: Concept, Rationale, and Design. J Am Geriatr Soc. 2017.
32. Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864-71.
33. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116(8):1413-30.
34. Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, et al. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol. 2015;65(2):125-32.
35. Golpanian S, DiFede DL, Khan A, Schulman IH, Landin AM, Tompkins BA, et al. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. J Gerontol A Biol Sci Med Sci. 2017.
36. Golpanian S, DiFede DL, Pujol MV, Lowery MH, Levis-Dusseau S, Goldstein BJ, et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget. 2016;7(11):11899-912.
37. Tompkins BA, DiFede DL, Khan A, Landin AM, Schulman IH, Pujol MV, et al. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci. 2017;72(11):1513-22.
38. Sanders JL, Boudreau RM, Penninx BW, Simonsick EM, Kritchevsky SB, Satterfield S, et al. Association of a Modified Physiologic Index with mortality and incident disability: the Health, Aging, and Body Composition study. J Gerontol A Biol Sci Med Sci. 2012;67(12):1439-46.
39. Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med. 2012;172(15):1162-8.
40. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194-217.
41. Hsu FC, Kritchevsky SB, Liu Y, Kanaya A, Newman AB, Perry SE, et al. Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol A Biol Sci Med Sci. 2009;64(5):581-9.
42. Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726-34.